Systemic and uteroplacental renin--angiotensin system in normal and pre-eclamptic pregnancies
- PMID: 19124433
- PMCID: PMC2692864
- DOI: 10.1177/1753944708094529
Systemic and uteroplacental renin--angiotensin system in normal and pre-eclamptic pregnancies
Abstract
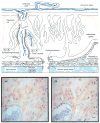
Pregnancy is characterized by an increase in many of the different components of the circulating renin-angiotensin system (RAS). However, the physiological mechanisms of stimulated RAS activity during pregnancy are unknown. Even less understood is how this system may be altered in pre-eclampsia, a hypertensive disorder of pregnancy. Additional studies have shown the presence of a local tissue specific RAS in the uteroplacental unit of normal and pre-eclamptic pregnancies. Differences in normal pregnant and pre-eclamptic RAS component regulation may provide insight into the mechanisms responsible for the clinical pathological features of pre-eclampsia. Specifically, this review summarizes the key findings in the circulating and uteroplacental RAS in normal and pre-eclamptic pregnancies.
Figures




Similar articles
-
The uteroplacental renin-angiotensin system: a review.Exp Clin Endocrinol. 1994;102(3):252-61. doi: 10.1055/s-0029-1211289. Exp Clin Endocrinol. 1994. PMID: 7995347 Review.
-
Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia.Hypertension. 2007 Mar;49(3):604-11. doi: 10.1161/01.HYP.0000257797.49289.71. Epub 2007 Jan 29. Hypertension. 2007. PMID: 17261642
-
Current topic: the uteroplacental renin-angiotensin system.Placenta. 2000 Jul-Aug;21(5-6):468-77. doi: 10.1053/plac.2000.0535. Placenta. 2000. PMID: 10940196 Review.
-
Alterations in the 'local umbilical cord blood renin-angiotensin system' during pre-eclampsia.Acta Obstet Gynecol Scand. 2007 Oct;86(10):1193-9. doi: 10.1080/00016340701552434. Acta Obstet Gynecol Scand. 2007. PMID: 17851801
-
The functional role of the renin-angiotensin system in pregnancy and preeclampsia.Placenta. 2008 Sep;29(9):763-71. doi: 10.1016/j.placenta.2008.06.011. Epub 2008 Aug 8. Placenta. 2008. PMID: 18687466 Free PMC article. Review.
Cited by
-
Associations of ACE I/D, AGT M235T gene polymorphisms with pregnancy induced hypertension in Chinese population: a meta-analysis.J Assist Reprod Genet. 2012 Sep;29(9):921-32. doi: 10.1007/s10815-012-9800-4. Epub 2012 May 30. J Assist Reprod Genet. 2012. PMID: 22644634 Free PMC article.
-
Maternal endothelial dysfunction in HIV-associated preeclampsia comorbid with COVID-19: a review.Hypertens Res. 2021 Apr;44(4):386-398. doi: 10.1038/s41440-020-00604-y. Epub 2021 Jan 20. Hypertens Res. 2021. PMID: 33469197 Free PMC article. Review.
-
Candidate Gene, Genome-Wide Association and Bioinformatic Studies in Pre-eclampsia: a Review.Curr Hypertens Rep. 2018 Aug 29;20(10):91. doi: 10.1007/s11906-018-0891-x. Curr Hypertens Rep. 2018. PMID: 30159611 Review.
-
Maternal Venous Hemodynamic Dysfunction in Proteinuric Gestational Hypertension: Evidence and Implications.J Clin Med. 2019 Mar 11;8(3):335. doi: 10.3390/jcm8030335. J Clin Med. 2019. PMID: 30862007 Free PMC article. Review.
-
Different effects of tocolytic medication on blood pressure and blood pressure amplification.Eur J Clin Pharmacol. 2011 Jan;67(1):11-7. doi: 10.1007/s00228-010-0926-y. Epub 2010 Nov 16. Eur J Clin Pharmacol. 2011. PMID: 21079937 Clinical Trial.
References
-
- Abbas A, Gorelik G, Carbini LA, Scicli AG. Angiotensin-(1-7) induces bradykinin-mediated hypotensive responses in anesthetized rats. Hypertension. 1997;30:217–221. - PubMed
-
- Alhenc-Gelas R, Tache A, Saint-Andre JP, Milliez J, Sureau C, Corvol P, et al. The Renin–Angiotensin System in Pregnancy and Parturition. Adv Nephrol Necker Hoap. 1986;15:25–33. - PubMed
-
- Allred AJ, Diz DI, Ferrarrio CM, Chappell MC. Pathways for angiotensin-(1-7) metabolism in pulmonary and renal tissues. Am J Physiol. 2000;279:F841–F850. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

