Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors
- PMID: 19124524
- PMCID: PMC2770104
- DOI: 10.1136/ard.2008.101121
Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors
Abstract
Objectives: To assess the efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis (RA) and poor prognostic factors.
Methods: In this double-blind, phase IIIb study, patients with RA for 2 years or less were randomly assigned 1 : 1 to receive abatacept (approximately 10 mg/kg) plus methotrexate, or placebo plus methotrexate. Patients were methotrexate-naive and seropositive for rheumatoid factor (RF), anti-cyclic citrullinated protein (CCP) type 2 or both and had radiographic evidence of joint erosions. The co-primary endpoints were the proportion of patients achieving disease activity score in 28 joints (DAS28)-defined remission (C-reactive protein) and joint damage progression (Genant-modified Sharp total score; TS) at year 1. Safety was monitored throughout.
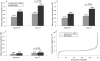
Results: At baseline, patients had a mean DAS28 of 6.3, a mean TS of 7.1 and mean disease duration of 6.5 months; 96.5% and 89.0% of patients were RF or anti-CCP2 seropositive, respectively. At year 1, a significantly greater proportion of abatacept plus methotrexate-treated patients achieved remission (41.4% vs 23.3%; p<0.001) and there was significantly less radiographic progression (mean change in TS 0.63 vs 1.06; p = 0.040) versus methotrexate alone. Over 1 year, the frequency of adverse events (84.8% vs 83.4%), serious adverse events (7.8% vs 7.9%), serious infections (2.0% vs 2.0%), autoimmune disorders (2.3% vs 2.0%) and malignancies (0.4% vs 0%) was comparable for abatacept plus methotrexate versus methotrexate alone.
Conclusions: In a methotrexate-naive population with early RA and poor prognostic factors, the combination of abatacept and methotrexate provided significantly better clinical and radiographic efficacy compared with methotrexate alone and had a comparable, favourable safety profile.
Conflict of interest statement
Competing interests: RW has received consulting fees and speaker’s bureau fees from Bristol-Myers Squibb and Schering-Plough and research support from UCB; ACX was a member of the national board as a consultant for Bristol-Myers Squibb (2003–7) and Abbott (2003–5); JW has received consulting fees from Bristol-Myers Squibb; JG-R is a member of advisory boards for Wyeth, Schering-Plough, Bristol-Myers Squibb and Roche and has received lecture fees from Abbott, Wyeth, Roche, Bristol-Myers Squibb and Schering-Plough; WG is a consultant for Bristol-Myers Squibb, Abbott Immunology, General Electric, Esaote and Schering-Plough, has received honorarium from Bristol-Myers Squibb, Abbott Immunology, General Electric, Schering-Plough and Wyeth and has received research support from Abbott Immunology and Wyeth; BH is a consultant and has received a grant/research support for Abbott Canada, Amgen Canada, Bristol-Myers Squibb Canada, Roche and Schering-Plough; WS is a speaker and principal investigator for Amgen, Wyeth, Abbott, Bristol-Myers Squibb, Centocor, Genentech and Biogen Idec; HG is a consultant for CCBR-SYNARC, Bristol-Myers Squibb, Wyeth, Roche, Servier, GlaxoSmithKline, Merck, Biogen Idec and Genentech and has stocks in CCBR-SYNARC; CP is an employee and has stocks in Synarc Inc; J-CB is an employee of BMS and owns stock options; AC and RH are employees of Bristol-Myers Squibb; JB is a consultant for Centocor and Riley, is on the advisory board for Abbott and Amgen, has received research support for Amgen, Biogen Idec and Bristol-Myers Squibb and has received honorarium for Abbott, Amgen, Centocor and Novartis. MR, SN, PD and SHP have nothing to disclose.
Figures


References
-
- Kroot EJ, de Jong BA, van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 2000;43:1831–5 - PubMed
-
- Vittecoq O, Pouplin S, Krzanowska K, et al. Rheumatoid factor is the strongest predictor of radiological progression of rheumatoid arthritis in a three-year prospective study in community-recruited patients. Rheumatology (Oxford) 2003;42:939–46 - PubMed
-
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90 - PubMed
-
- Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

