Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4
- PMID: 19124557
- PMCID: PMC2669421
- DOI: 10.1096/fj.08-118323
Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4
Abstract
Ajulemic acid (AjA), a synthetic nonpsychoactive cannabinoid, and lipoxin A(4) (LXA(4)), an eicosanoid formed from sequential actions of 5- and 15-lipoxygenases (LOX), facilitate resolution of inflammation. The purpose of this study was to determine whether the ability of AjA to limit the progress of inflammation might relate to an increase in LXA(4), a known anti-inflammatory and proresolving mediator. Addition of AjA (0-30 microM) in vitro to human blood and synovial cells increased production of LXA(4) (ELISA) 2- to 5-fold. Administration of AjA to mice with peritonitis resulted in a 25-75% reduction of cells invading the peritoneum, and a 7-fold increase in LXA(4) identified by mass spectrometry. Blockade of 12/15 LOX, which leads to LXA(4) synthesis via 15-HETE production, reduced (>90%) the ability of AjA to enhance production of LXA(4) in vitro. These results suggest that AjA and other agents that increase endogenous compounds that facilitate resolution of inflammation may be useful for conditions characterized by inflammation and tissue injury.
Figures

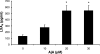



Similar articles
-
Suppression of human monocyte interleukin-1beta production by ajulemic acid, a nonpsychoactive cannabinoid.Biochem Pharmacol. 2003 Feb 15;65(4):649-55. doi: 10.1016/s0006-2952(02)01604-0. Biochem Pharmacol. 2003. PMID: 12566094
-
Suppression of fibroblast metalloproteinases by ajulemic acid, a nonpsychoactive cannabinoid acid.J Cell Biochem. 2007 Jan 1;100(1):184-90. doi: 10.1002/jcb.21046. J Cell Biochem. 2007. PMID: 16927387
-
Ajulemic acid, a synthetic cannabinoid acid, induces an antiinflammatory profile of eicosanoids in human synovial cells.Life Sci. 2008 Nov 7;83(19-20):666-70. doi: 10.1016/j.lfs.2008.09.004. Epub 2008 Sep 21. Life Sci. 2008. PMID: 18840450
-
PPAR-gamma: a nuclear receptor with affinity for cannabinoids.Life Sci. 2005 Aug 19;77(14):1674-84. doi: 10.1016/j.lfs.2005.05.039. Life Sci. 2005. PMID: 16005906 Review.
-
The cannabinoid acids, analogs and endogenous counterparts.Bioorg Med Chem. 2014 May 15;22(10):2830-43. doi: 10.1016/j.bmc.2014.03.038. Epub 2014 Apr 1. Bioorg Med Chem. 2014. PMID: 24731541 Free PMC article. Review.
Cited by
-
Cannabinoid exposure during pregnancy and its impact on immune function.Cell Mol Life Sci. 2019 Feb;76(4):729-743. doi: 10.1007/s00018-018-2955-0. Epub 2018 Oct 29. Cell Mol Life Sci. 2019. PMID: 30374520 Free PMC article. Review.
-
Safety and Efficacy of Lenabasum, a Cannabinoid Receptor Type 2 Agonist, in Patients with Dermatomyositis with Refractory Skin Disease: A Randomized Clinical Trial.J Invest Dermatol. 2022 Oct;142(10):2651-2659.e1. doi: 10.1016/j.jid.2022.03.029. Epub 2022 Apr 29. J Invest Dermatol. 2022. PMID: 35490744 Free PMC article. Clinical Trial.
-
Potent Anti-Inflammatory and Pro-Resolving Effects of Anabasum in a Human Model of Self-Resolving Acute Inflammation.Clin Pharmacol Ther. 2018 Oct;104(4):675-686. doi: 10.1002/cpt.980. Epub 2018 Jan 30. Clin Pharmacol Ther. 2018. PMID: 29238967 Free PMC article. Clinical Trial.
-
Therapeutic potential and safety considerations for the clinical use of synthetic cannabinoids.Pharmacol Biochem Behav. 2020 Dec;199:173059. doi: 10.1016/j.pbb.2020.173059. Epub 2020 Oct 18. Pharmacol Biochem Behav. 2020. PMID: 33086126 Free PMC article. Review.
-
Roles, Actions, and Therapeutic Potential of Specialized Pro-resolving Lipid Mediators for the Treatment of Inflammation in Cystic Fibrosis.Front Pharmacol. 2019 Apr 2;10:252. doi: 10.3389/fphar.2019.00252. eCollection 2019. Front Pharmacol. 2019. PMID: 31001110 Free PMC article. Review.
References
-
- Abel E L. New York, NY, USA: Plenum Press; MarijuanaThe First Thousand Years. 1980
-
- Burstein S H, Rosenfeld J, Wittstruck T. Isolation and characterization of two major urinary metabolites of Δ1-tetrahydrocannabinol. Science. 1972;176:422–424. - PubMed
-
- Perez-Reyes M. Pharmacodynamics of certain drugs of abuse. Barnett G, Chiang N C, editors. Foster City, CA, USA: Biomedical Publishers; Pharmacokinetics and Pharmacodynamics of Psychotropic Drugs. 1985:287–310.
-
- Loev B, Bender P E, Dowalo F, Macko E, Fowler P. Cannabinoids: structure-activity studies related to 1,2-dimethylheptyl derivatives. J Med Chem. 1993;16:1200–1206. - PubMed
-
- Burstein S H, Audette C, Breuer A, Devane W A, Colodner S, Doyle S A, Mechoulam R. Synthetic nonpsychotropic cannabinoids with potent antiinflammatory, analgesic and leukocyte anti-adhesion properties. J Med Chem. 1992;35:3135–3141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

