Tropomyosin isoform expression regulates the transition of adhesions to determine cell speed and direction
- PMID: 19124607
- PMCID: PMC2648248
- DOI: 10.1128/MCB.00857-08
Tropomyosin isoform expression regulates the transition of adhesions to determine cell speed and direction
Abstract
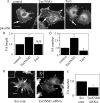
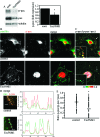
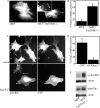
The balance of transition between distinct adhesion types contributes to the regulation of mesenchymal cell migration, and the characteristic association of adhesions with actin filaments led us to question the role of actin filament-associating proteins in the transition between adhesive states. Tropomyosin isoform association with actin filaments imparts distinct filament structures, and we have thus investigated the role for tropomyosins in determining the formation of distinct adhesion structures. Using combinations of overexpression, knockdown, and knockout approaches, we establish that Tm5NM1 preferentially stabilizes focal adhesions and drives the transition to fibrillar adhesions via stabilization of actin filaments. Moreover, our data suggest that the expression of Tm5NM1 is a critical determinant of paxillin phosphorylation, a signaling event that is necessary for focal adhesion disassembly. Thus, we propose that Tm5NM1 can regulate the feedback loop between focal adhesion disassembly and focal complex formation at the leading edge that is required for productive and directed cell movement.
Figures






References
-
- Bargon, S. D., P. W. Gunning, and G. M. O'Neill. 2005. The Cas family docking protein, HEF1, promotes the formation of neurite-like membrane extensions. Biochim. Biophys. Acta 1 746143-154. - PubMed
-
- Bryce, N. S., G. Schevzov, V. Ferguson, J. M. Percival, J. J. Lin, F. Matsumura, J. R. Bamburg, P. L. Jeffrey, E. C. Hardeman, P. Gunning, and R. P. Weinberger. 2003. Specification of actin filament function and molecular composition by tropomyosin isoforms. Mol. Biol. Cell 141002-1016. - PMC - PubMed
-
- Carragher, N. O., and M. C. Frame. 2004. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 14241-249. - PubMed
-
- Cau, J., and A. Hall. 2005. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell Sci. 1182579-2587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
