Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling
- PMID: 19124657
- PMCID: PMC2626688
- DOI: 10.1084/jem.20082058
Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling
Abstract
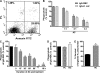
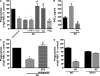
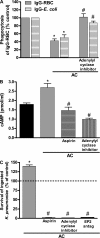
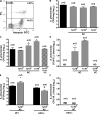
The ingestion of apoptotic cells (ACs; termed "efferocytosis") by phagocytes has been shown to trigger the release of molecules such as transforming growth factor beta, interleukin-10 (IL-10), nitric oxide, and prostaglandin E(2) (PGE(2)). Although the antiinflammatory actions of these mediators may contribute to the restoration of homeostasis after tissue injury, their potential impact on antibacterial defense is unknown. The lung is highly susceptible to diverse forms of injury, and secondary bacterial infections after injury are of enormous clinical importance. We show that ACs suppress in vitro phagocytosis and bacterial killing by alveolar macrophages and that this is mediated by a cyclooxygenase-PGE(2)-E prostanoid receptor 2 (EP2)-adenylyl cyclase-cyclic AMP pathway. Moreover, intrapulmonary administration of ACs demonstrated that PGE(2) generated during efferocytosis and acting via EP2 accounts for subsequent impairment of lung recruitment of polymorphonuclear leukocytes and clearance of Streptococcus pneumoniae, as well as enhanced generation of IL-10 in vivo. These results suggest that in addition to their beneficial homeostatic influence, antiinflammatory programs activated by efferocytosis in the lung have the undesirable potential to dampen innate antimicrobial responses. They also identify an opportunity to reduce the incidence and severity of pneumonia in the setting of lung injury by pharmacologically targeting synthesis of PGE(2) or ligation of EP2.
Figures
References
-
- Wheeler, A.P., and G.R. Bernard. 2007. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 369:1553–1564. - PubMed
-
- Fadok, V.A., D.L. Bratton, A. Konowal, P.W. Freed, J.Y. Westcott, and P.M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101:890–898. - PMC - PubMed
-
- Voll, R.E., M. Herrmann, E.A. Roth, C. Stach, J.R. Kalden, and I. Girkontaite. 1997. Immunosuppressive effects of apoptotic cells. Nature. 390:350–351. - PubMed
-
- McDonald, P.P., V.A. Fadok, D. Bratton, and P.M. Henson. 1999. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J. Immunol. 163:6164–6172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

