Tuberculosis-induced variant IL-4 mRNA encodes a cytokine functioning as growth factor for (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-specific Vgamma2Vdelta2 T cells
- PMID: 19124724
- PMCID: PMC2743098
- DOI: 10.4049/jimmunol.182.2.811
Tuberculosis-induced variant IL-4 mRNA encodes a cytokine functioning as growth factor for (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-specific Vgamma2Vdelta2 T cells
Abstract
The possibility that mycobacterial infections induce variant cytokine mRNA encoding a functionally distinct protein for immune regulation has not been addressed. In this study, we reported that Mycobacterium tuberculosis and bacillus Calmette-Guérin infections of macaques induced expression of variant IL-4 (VIL-4) mRNA encoding a protein comprised of N-terminal 97 aa identical with IL-4, and unique C-terminal 96 aa including a signaling-related proline-rich motif. While VIL-4 could be stably produced as intact protein, the purified VIL-4 induced apparent expansion of phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP)-specific Vgamma2Vdelta2 T cells in dose- and time-dependent manners. The unique C-terminal 96 aa bearing the proline-rich motif (PPPCPP) of VIL-4 appeared to confer the ability to expand Vgamma2Vdelta2 T cells, since simultaneously produced IL-4 had only a subtle effect on these gammadelta T cells. Moreover, VIL-4 seemed to use IL-4R alpha for signaling and activation, as the VIL-4-induced expansion of Vgamma2Vdelta2 T cells was blocked by anti-IL-4R alpha mAb but not anti-IL-4 mAb. Surprisingly, VIL-4-expanded Vgamma2Vdelta2 T cells after HMBPP stimulation appeared to be heterologous effector cells capable of producing IL-4, IFN-gamma, and TNF-alpha. Thus, mycobacterial infections of macaques induced variant mRNA encoding VIL-4 that functions as growth factor promoting expansion of HMBPP-specific Vgamma2Vdelta2 T effector cells.
Figures


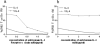

References
-
- Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γ δ T cells in vivo. Nature. 1995;373:255–257. - PubMed
-
- Brown MA, Pierce JH, Watson CJ, Falco J, Ihle JN, Paul WE. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987;50:809–818. - PubMed
-
- MacGlashan D, Jr, White JM, Huang SK, Ono SJ, Schroeder JT, Lichtenstein LM. Secretion of IL-4 from human basophils: the relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol. 1994;152:3006–3016. - PubMed
-
- Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–1870. - PubMed
-
- Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

