Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells
- PMID: 19124755
- PMCID: PMC3682801
- DOI: 10.4049/jimmunol.182.2.1119
Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells
Abstract
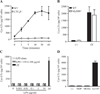
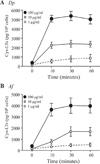
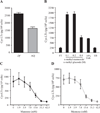
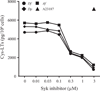
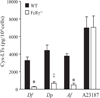
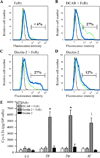
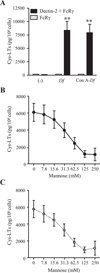
House dust mites are a significant source of airborne allergen worldwide, but there is little understanding of how they so potently generate allergic inflammation. We found that extracts from the house dust mites Dermatophagoides farinae (Df) and Dermatophagoides pteronyssinus and from the mold Aspergillus fumigatus stimulated a rapid and robust production of cysteinyl leukotrienes (cys-LTs), proinflammatory lipid mediators, from mouse bone marrow-derived dendritic cells (BMDCs). Con A affinity chromatography of the Df extract revealed that the relevant ligand is a glycan(s), suggesting stimulation via a dendritic cell (DC) lectin receptor. Cys-LT production in BMDCs from wild-type mice was inhibited by spleen tyrosine kinase (Syk) inhibitors and was abolished in BMDCs from FcRgamma-/- mice, implicating either Dectin-2 or DC immunoactivating receptor. Transfection of each receptor in bone marrow-derived mast cells revealed that only Dectin-2 mediates cys-LT production by Df, Dermatophagoides pteronyssinus, and Aspergillus fumigatus. Lentiviral knockdown of Dectin-2 in BMDCs attenuated Df extract-elicited cys-LT generation, thereby identifying Dectin-2 as the receptor. Lung CD11c+ cells, but not peritoneal or alveolar macrophages, also generated cys-LTs in response to Df. These findings place Dectin-2 among the C-type lectin receptors that activate arachidonic acid metabolism and identify the Dectin-2/FcRgamma/Syk/cys-LT axis as a novel mechanism by which three potent indoor allergens may activate innate immune cells to promote allergic inflammation.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures
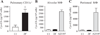

Comment in
-
A novel non-IgE-mediated pathway of mite-induced inflammation.J Allergy Clin Immunol. 2010 Aug;126(2):403-4. doi: 10.1016/j.jaci.2010.05.019. J Allergy Clin Immunol. 2010. PMID: 20619884 No abstract available.
References
-
- Suram S, Brown GD, Ghosh M, Gordon S, Loper R, Taylor PR, Akira S, Uematsu S, Williams DL, Leslie CC. Regulation of cytosolic phospholipase A2 activation and cyclooxygenase 2 expression in macrophages by the beta-glucan receptor. J. Biol. Chem. 2006;281:5506–5514. - PubMed
-
- Valera I, Fernandez N, Trinidad AG, Alonso S, Brown GD, Alonso A, Crespo MS. Costimulation of dectin-1 and DC-SIGN triggers the arachidonic acid cascade in human monocyte-derived dendritic cells. J. Immunol. 2008;180:5727–5736. - PubMed
-
- van Vliet SJ, den Dunnen J, Gringhuis SI, Geijtenbeek TB, van Kooyk Y. Innate signaling and regulation of dendritic cell immunity. Curr. Opin. Immunol. 2007;19:435–440. - PubMed
-
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. - PubMed
-
- Austen KF. The cysteinyl leukotrienes: where do they come from? What are they? Where are they going? Nat. Immunol. 2008;9:113–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL036110/HL/NHLBI NIH HHS/United States
- U19 AI031599/AI/NIAID NIH HHS/United States
- K08 AI080948/AI/NIAID NIH HHS/United States
- T32 AI007306/AI/NIAID NIH HHS/United States
- HL-82695/HL/NHLBI NIH HHS/United States
- R01 HL082695/HL/NHLBI NIH HHS/United States
- AI-52353/AI/NIAID NIH HHS/United States
- R56 AI052353/AI/NIAID NIH HHS/United States
- R37 AI052353/AI/NIAID NIH HHS/United States
- P01 AI031599/AI/NIAID NIH HHS/United States
- AI-31599/AI/NIAID NIH HHS/United States
- R01 AI052353/AI/NIAID NIH HHS/United States
- AI-07306/AI/NIAID NIH HHS/United States
- HL-36110/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

