Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis
- PMID: 19124768
- PMCID: PMC2613937
- DOI: 10.1073/pnas.0810249106
Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis
Abstract
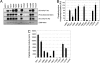
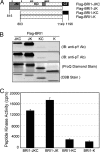
Brassinosteroids (BRs) are essential growth-promoting hormones that regulate many aspects of plant growth and development. Two leucine-rich repeat receptor-like kinases (LRR-RLKs) are involved in BR perception and signal transduction: brassinosteroid insensitive 1 (BRI1), which is the BR receptor, and its coreceptor BRI1-associated kinase 1 (BAK1). Both proteins are classified as serine/threonine protein kinases, but here we report that recombinant cytoplasmic domains of BRI1 and BAK1 also autophosphorylate on tyrosine residues and thus are dual-specificity kinases. With BRI1, Tyr-831 and Tyr-956 are identified as autophosphorylation sites in vitro and in vivo. Interestingly, Tyr-956 in kinase subdomain V is essential for activity, because the Y956F mutant is catalytically inactive and thus this site cannot be simply manipulated by mutagenesis. In contrast, Tyr-831 in the juxtamembrane domain is not essential for kinase activity but plays an important role in BR signaling in vivo, because expression of BRI1(Y831F)-Flag in transgenic bri1-5 plants results in plants with larger leaves (but altered leaf shape) and early flowering relative to plants expressing wild-type BRI1-Flag. Acidic substitutions of Tyr-831 restored normal leaf size (but not shape) and normal flowering time. This is an example where a specific tyrosine residue has been shown to play an important role in vivo in plant receptor kinase function. Interestingly, 6 additional LRR-RLKs (of the 23 tested) were also found to autophosphorylate on tyrosine in addition to serine and threonine, suggesting that tyrosine signaling should be considered with other plant receptor kinases as well.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Tyrosine phosphorylation in brassinosteroid signaling.Plant Signal Behav. 2009 Dec;4(12):1182-5. doi: 10.4161/psb.4.12.10046. Plant Signal Behav. 2009. PMID: 20514242 Free PMC article.
References
-
- Kinoshita T, et al. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature. 2005;433:167–171. - PubMed
-
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. - PubMed
-
- Li J, et al. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. - PubMed
-
- Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

