Efficacy of pneumococcal vaccination in adults: a meta-analysis
- PMID: 19124790
- PMCID: PMC2612051
- DOI: 10.1503/cmaj.080734
Efficacy of pneumococcal vaccination in adults: a meta-analysis
Erratum in
- CMAJ. 2009 May 12;180(10):1038
Abstract
Background: Clinical trials and meta-analyses have produced conflicting results of the efficacy of unconjugated pneumococcal polysaccharide vaccine in adults. We sought to evaluate the vaccine's efficacy on clinical outcomes as well as the methodologic quality of the trials.
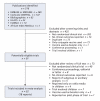
Methods: We searched several databases and all bibliographies of reviews and meta-analyses for clinical trials that compared pneumococcal polysaccharide vaccine with a control. We examined rates of pneumonia and death, taking the methodologic quality of the trials into consideration.
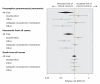
Results: We included 22 trials involving 101 507 participants: 11 trials reported on presumptive pneumococcal pneumonia, 19 on all-cause pneumonia and 12 on all-cause mortality. The current 23-valent vaccine was used in 8 trials. The relative risk (RR) was 0.64 (95% confidence interval [CI] 0.43-0.96) for presumptive pneumococcal pneumonia and 0.73 (95% CI 0.56-0.94) for all-cause pneumonia. There was significant heterogeneity between the trials reporting on presumptive pneumonia (I(2) = 74%, p < 0.001) and between those reporting on all-cause pneumonia (I(2) = 90%, p < 0.001). The RR for all-cause mortality was 0.97 (95% CI 0.87-1.09), with moderate heterogeneity between trials (I(2) = 44%, p = 0.053). Trial quality, especially regarding double blinding, explained a substantial proportion of the heterogeneity in the trials reporting on presumptive pneumonia and all-cause pneumonia. There was little evidence of vaccine protection in trials of higher methodologic quality (RR 1.20, 95% CI 0.75-1.92, for presumptive pneumonia; and 1.19, 95% CI 0.95-1.49, for all-cause pneumonia in double-blind trials; p for heterogeneity > 0.05). The results for all-cause mortality in double-blind trials were similar to those in all trials combined. There was little evidence of vaccine protection among elderly patients or adults with chronic illness in analyses of all trials (RR 1.04, 95% CI 0.78-1.38, for presumptive pneumococcal pneumonia; 0.89, 95% CI 0.69-1.14, for all-cause pneumonia; and 1.00, 95% CI 0.87-1.14, for all-cause mortality).
Interpretation: Pneumococcal vaccination does not appear to be effective in preventing pneumonia, even in populations for whom the vaccine is currently recommended.
Figures

Comment in
-
The controversy over the efficacy of pneumococcal vaccine.CMAJ. 2009 Jan 6;180(1):18-9. doi: 10.1503/cmaj.081744. CMAJ. 2009. PMID: 19124781 Free PMC article. No abstract available.
-
Review: pneumococcal vaccination does not prevent pneumonia, bacteraemia, bronchitis, or mortality.Evid Based Nurs. 2009 Jul;12(3):74. doi: 10.1136/ebn.12.3.74. Evid Based Nurs. 2009. PMID: 19553411 No abstract available.
-
Review: pneumococcal vaccination is not effective for preventing pneumonia, bacteraemia, bronchitis, or mortality.Evid Based Med. 2009 Aug;14(4):109. doi: 10.1136/ebm.14.4.109. Evid Based Med. 2009. PMID: 19648425 No abstract available.
References
-
- Noakes K, Pebody RG, Gungabissoon U, et al. Pneumococcal polysaccharide vaccine uptake in England, 1989–2003, prior to the introduction of a vaccination programme for older adults. J Public Health (Oxf) 2006;28:242-7. - PubMed
-
- Joint Committee on Vaccination and Immunisation, UK Department of Health. Minutes of the meeting held on Friday 3 May 2002 at 10.30. London (UK): The Department; 2002 Dec 18. Available: www.advisorybodies.doh.gov.uk/jcvi/mins03may03.htm (accessed 2008 4 Dec).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
