Recommendations from the EGAPP Working Group: can tumor gene expression profiling improve outcomes in patients with breast cancer?
- PMID: 19125125
- PMCID: PMC2743614
- DOI: 10.1097/GIM.0b013e3181928f56
Recommendations from the EGAPP Working Group: can tumor gene expression profiling improve outcomes in patients with breast cancer?
Abstract
Summary of recommendations: The EGAPP Working Group (EWG) found insufficient evidence to make a recommendation for or against the use of tumor gene expression profiles to improve outcomes in defined populations of women with breast cancer. For one test, the EWG found preliminary evidence of potential benefit of testing results to some women who face decisions about treatment options (reduced adverse events due to low risk women avoiding chemotherapy), but could not rule out the potential for harm for others (breast cancer recurrence that might have been prevented). The evidence is insufficient to assess the balance of benefits and harms of the proposed uses of the tests. The EWG encourages further development and evaluation of these technologies.
Rationale: The measurement of gene expression in breast tumor tissue is proposed as a way to estimate the risk of distant disease recurrence in order to provide additional information beyond current clinicopathological risk stratification and to influence decisions about treatment in order to improve health outcomes. Based on their review of the EGAPP-commissioned evidence report, Impact of Gene Expression Profiling Tests on Breast Cancer Outcomes and other data summaries, the EWG found no direct evidence linking tumor gene expression profiling of women with breast cancer to improved outcomes, and inadequate evidence to construct an evidence chain. However, further evaluation on the clinical utility of some tests and management algorithms, including well-designed randomized controlled trials, is warranted.
Analytic validity: Some data on technical performance of assays were identified for MammaPrint and Oncotype DX, though estimates of analytic sensitivity and specificity could not be made. Published performance data on the laboratory developed Quest H:I Test were limited. Overall, the EWG found the evidence to be inadequate.
Clinical validity: The EWG found adequate evidence regarding the association of the Oncotype DX Recurrence Score with disease recurrence and adequate evidence for response to chemotherapy. The EWG found adequate evidence to characterize the association of MammaPrint with future metastases, but inadequate evidence to assess the added value to standard risk stratification, and could not determine the population to which the test would best apply. The evidence was inadequate to characterize the clinical validity of the Quest H:I Test.
Clinical utility: The EWG found no evidence regarding the clinical utility of the MammaPrint and Quest H:I Ratio tests, and inadequate evidence regarding Oncotype DX. These technologies have potential for both benefit and harm.
Contextual issues: The EWG reviewed economic studies that used modeling to predict potential effects of using gene profiling, and judged the evidence inadequate.
Figures
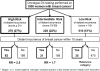
References
-
- Carlson RW, Brown E, Burstein HJ, et al. for the National Comprehensive Cancer Network. NCCN task force report: adjuvant therapy for breast cancer. J Natl Compr Canc Netw. 2006;4(suppl 1):S1–S26. - PubMed
-
- Adjuvant! Online. [Accessed August 2008]. Available at: https:// www.adjuvantonline.com/faq.jsp.
-
- Harbeck N, Jakesz R. St. Gallen 2007: Breast Cancer Treatment Consensus Report. Breast Care. 2007;2:130–134.
-
- Marchionni L, Wilson RF, Wolff AC, et al. Systematic review: gene expression profiling assays in early-stage breast cancer. Ann Intern Med. 2008;148:358–369. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

