Ancestral vascular lumen formation via basal cell surfaces
- PMID: 19125185
- PMCID: PMC2607016
- DOI: 10.1371/journal.pone.0004132
Ancestral vascular lumen formation via basal cell surfaces
Abstract
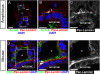
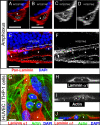
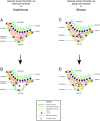
The cardiovascular system of bilaterians developed from a common ancestor. However, no endothelial cells exist in invertebrates demonstrating that primitive cardiovascular tubes do not require this vertebrate-specific cell type in order to form. This raises the question of how cardiovascular tubes form in invertebrates? Here we discovered that in the invertebrate cephalochordate amphioxus, the basement membranes of endoderm and mesoderm line the lumen of the major vessels, namely aorta and heart. During amphioxus development a laminin-containing extracellular matrix (ECM) was found to fill the space between the basal cell surfaces of endoderm and mesoderm along their anterior-posterior (A-P) axes. Blood cells appear in this ECM-filled tubular space, coincident with the development of a vascular lumen. To get insight into the underlying cellular mechanism, we induced vessels in vitro with a cell polarity similar to the vessels of amphioxus. We show that basal cell surfaces can form a vascular lumen filled with ECM, and that phagocytotic blood cells can clear this luminal ECM to generate a patent vascular lumen. Therefore, our experiments suggest a mechanism of blood vessel formation via basal cell surfaces in amphioxus and possibly in other invertebrates that do not have any endothelial cells. In addition, a comparison between amphioxus and mouse shows that endothelial cells physically separate the basement membranes from the vascular lumen, suggesting that endothelial cells create cardiovascular tubes with a cell polarity of epithelial tubes in vertebrates and mammals.
Conflict of interest statement
Figures


References
-
- Bishopric NH. Evolution of the heart from bacteria to man. Ann N Y Acad Sci. 2005;1047:13–29. - PubMed
-
- Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–690. - PubMed
-
- Hartenstein V, Mandal L. The blood/vascular system in a phylogenetic perspective. Bioessays. 2006;28:1203–1210. - PubMed
-
- Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet. 2004;36:1019–1023. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

