Radioimmunoimaging with longer-lived positron-emitting radionuclides: potentials and challenges
- PMID: 19125647
- PMCID: PMC3397469
- DOI: 10.1021/bc800299f
Radioimmunoimaging with longer-lived positron-emitting radionuclides: potentials and challenges
Abstract
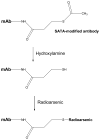
Radioimmunoimaging and therapy has been an area of interest for several decades. Steady progress has been made toward clinical translation of radiolabeled monoclonal antibodies for diagnosis and treatment of diseases. Tremendous advances have been made in imaging technologies such as positron emission tomography (PET). However, these advances have so far eluded routine translation into clinical radioimmunoimaging applications due to the mismatch between the short half-lives of routinely used positron-emitting radionuclides such as (18)F versus the pharmacokinetics of most intact monoclonal antibodies of interest. The lack of suitable positron-emitting radionuclides that match the pharmacokinetics of intact antibodies has generated interest in exploring the use of longer-lived positron emitters that are more suitable for radioimmunoimaging and dosimetry applications with intact monoclonal antibodies. In this review, we examine the opportunities and challenges of radioimmunoimaging with select longer-lived positron-emitting radionuclides such as (124)I, (89)Zr, and (86)Y with respect to radionuclide production, ease of radiolabeling intact antibodies, imaging characteristics, radiation dosimetry, and clinical translation potential.
Conflict of interest statement
Authors declare no conflict of interests.
Figures

References
-
- Cattell HW. Application of the X-Rays to Surgery. Science. 1896;3:344–346. - PubMed
-
- Wagner HN, Jr, Bardfeld PA. Evaluation of structure and function of spleen with radioactive tracers. JAMA. 1967;199:202–6. - PubMed
-
- Wagner HN., Jr The current status of nuclear medicine. JAMA. 1963;183:500–3. - PubMed
-
- Khaw BA. Antibodies for molecular imaging in the cardiovascular system. J Nucl Cardiol. 2005;12:591–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

