Tonabersat inhibits trigeminal ganglion neuronal-satellite glial cell signaling
- PMID: 19125874
- PMCID: PMC3138142
- DOI: 10.1111/j.1526-4610.2008.01262.x
Tonabersat inhibits trigeminal ganglion neuronal-satellite glial cell signaling
Abstract
Background: Sensitization and activation of trigeminal neurons are implicated in the underlying pathology of migraine, acute sinusitis, and allergic rhinitis. Cell bodies of trigeminal neurons that provide sensory innervation of the dura and nasal mucosa reside in the trigeminal ganglion in association with satellite glial cells where they communicate via gap junctions. Gap junctions, channels formed by connexins, modulate the excitability state of both neurons and glia under pathological conditions. Tonabersat, a compound being tested as an antimigraine drug, is thought to block gap junction activity.
Objective: To investigate the cellular events within trigeminal ganglia that may account for the significant comorbidity of migraine and rhinosinusitis and determine the effect of tonabersat on neuron-satellite glia communication.
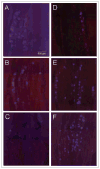
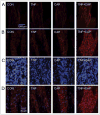
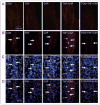
Methods: Sprague Dawley rats injected with True Blue were used to localize neuronal cell bodies in the ganglion and study neuron-glia signaling via gap junctions in the trigeminal ganglion. Dye coupling studies were conducted under basal conditions and in response to tumor necrosis factor-alpha injection into the whisker pad and/or capsaicin injection into the eyebrow. Changes in connexin 26 and active p38 levels were determined by immunohistochemistry. In addition, the effect of tonabersat prior to chemical stimulation on gap junction activity and expression of connexins and active p38 was investigated.
Results: Injection of tumor necrosis factor-alpha, a cytokine implicated in the pathology of acute sinusitis and allergic rhinitis, into the V2 region was shown to lower the amount of capsaicin required to stimulate neurons located in the V1 region of the ganglion. While injection of tumor necrosis factor-alpha into the whisker pad or capsaicin injection into the eyebrow alone did not cause increased dye movement, the combination of both stimuli greatly increased neuron-satellite glia communication via gap junctions in both V1 and V2 regions. The change in gap junction activity was accompanied by increased expression of connexin 26 and active p38 levels in both neurons and satellite glia in V1 and V2 regions. Pretreatment with tonabersat inhibited gap junction communication between neurons and satellite glia and blocked the increase in connexin 26 and active p38 levels in response to injection of both tumor necrosis factor-alpha (V2) and capsaicin (V1).
Conclusions: We propose that increased levels of tumor necrosis factor-alpha, as reported during acute sinusitis and allergic rhinitis, reduces the amount of capsaicin necessary to stimulate V1 neurons that leads to cellular changes in both V1 and V2 regions. The cellular events observed in this study may help to explain, in part, the significant comorbidity reported with migraine and rhinosinusitis. In addition, we have provided evidence to suggest that tonabersat can prevent increased neuron-satellite glia signaling and, thus, may be useful in the treatment of migraine, acute sinusitis, and allergic rhinitis.
Conflict of interest statement
Figures



Similar articles
-
Differential expression of connexins in trigeminal ganglion neurons and satellite glial cells in response to chronic or acute joint inflammation.Neuron Glia Biol. 2008 Nov;4(4):295-306. doi: 10.1017/S1740925X09990093. Epub 2009 Aug 13. Neuron Glia Biol. 2008. PMID: 19674505 Free PMC article.
-
Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology.Headache. 2007 Jul-Aug;47(7):1008-23; discussion 24-5. doi: 10.1111/j.1526-4610.2007.00854.x. Headache. 2007. PMID: 17635592 Free PMC article.
-
Neurological mechanisms of migraine: potential of the gap-junction modulator tonabersat in prevention of migraine.Cephalalgia. 2009 Nov;29 Suppl 2(Suppl 2):1-6. doi: 10.1111/j.1468-2982.2009.01976.x. Cephalalgia. 2009. PMID: 19723120 Free PMC article. Review.
-
Nitric oxide-proton stimulation of trigeminal ganglion neurons increases mitogen-activated protein kinase and phosphatase expression in neurons and satellite glial cells.Neuroscience. 2008 Dec 2;157(3):542-55. doi: 10.1016/j.neuroscience.2008.09.035. Epub 2008 Oct 1. Neuroscience. 2008. PMID: 18938228 Free PMC article.
-
[Gap junctional intercellular communication: a new mechanism in pathophysiology of migraine with aura. Therapeutic applications].Pathol Biol (Paris). 2012 Dec;60(6):392-8. doi: 10.1016/j.patbio.2012.04.002. Epub 2012 May 25. Pathol Biol (Paris). 2012. PMID: 22633071 Review. French.
Cited by
-
Future drugs for migraine.Intern Emerg Med. 2009 Oct;4(5):367-73. doi: 10.1007/s11739-009-0273-0. Epub 2009 Jun 24. Intern Emerg Med. 2009. PMID: 19551474 Review.
-
Glia and Orofacial Pain: Progress and Future Directions.Int J Mol Sci. 2021 May 19;22(10):5345. doi: 10.3390/ijms22105345. Int J Mol Sci. 2021. PMID: 34069553 Free PMC article. Review.
-
Development of functional units within trigeminal ganglia correlates with increased expression of proteins involved in neuron-glia interactions.Neuron Glia Biol. 2010 Aug;6(3):171-81. doi: 10.1017/S1740925X10000232. Epub 2010 Dec 16. Neuron Glia Biol. 2010. PMID: 21205366 Free PMC article.
-
An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy.Int J Mol Sci. 2021 Mar 21;22(6):3194. doi: 10.3390/ijms22063194. Int J Mol Sci. 2021. PMID: 33801118 Free PMC article. Review.
-
Differential expression of connexins in trigeminal ganglion neurons and satellite glial cells in response to chronic or acute joint inflammation.Neuron Glia Biol. 2008 Nov;4(4):295-306. doi: 10.1017/S1740925X09990093. Epub 2009 Aug 13. Neuron Glia Biol. 2008. PMID: 19674505 Free PMC article.
References
-
- Cady R, Schreiber C. Sinus headache or migraine? Considerations in making a differential diagnosis. Neurology. 2002;58:S10–14. - PubMed
-
- Schreiber C, Hutchinson S, Webster C, Ames M, Richardson M, Powers C. Prevalence of migraine in patients with a history of self-reported or physician-diagnosed “sinus” headache. Arch Intern Med. 2004;164:1769–1772. - PubMed
-
- Ku M, Silverman B, Prifti N, Ying W, Persaud Y, Schneider A. Prevalence of migraine headaches in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97:226–230. - PubMed
-
- Baraniuk J. Neurogenic mechanisms in rhinosinusitis. Curr Allergy Asthma Rep. 2001;1:252–261. - PubMed
-
- Goadsby P, Lipton R, Ferrari M. Migraine – current understanding and treatment. N Engl J Med. 2002;346:257–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

