T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity
- PMID: 19125887
- PMCID: PMC2632691
- DOI: 10.1111/j.1365-2567.2008.03015.x
T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity
Abstract
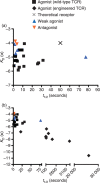
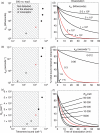
The interaction between the T-cell receptor (TCR) and its peptide-major histocompatibility complex (pepMHC) ligand plays a critical role in determining the activity and specificity of the T cell. The binding properties associated with these interactions have now been studied in many systems, providing a framework for a mechanistic understanding of the initial events that govern T-cell function. There have been various other reviews that have described the structural and biochemical features of TCR : pepMHC interactions. Here we provide an overview of four areas that directly impact our understanding of T-cell function, as viewed from the perspective of the TCR : pepMHC interaction: (1) relationships between T-cell activity and TCR : pepMHC binding parameters, (2) TCR affinity, avidity and clustering, (3) influence of coreceptors on pepMHC binding by TCRs and T-cell activity, and (4) impact of TCR binding affinity on antigenic peptide specificity.
Figures

References
-
- Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–66. - PubMed
-
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–44. - PubMed
-
- Slansky JE, Rattis FM, Boyd LF, Fahmy T, Jaffee EM, Schneck JP, Margulies DH, Pardoll DM. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13:529–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

