Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress
- PMID: 19126602
- PMCID: PMC2642602
- DOI: 10.1093/cvr/cvn360
Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress
Abstract
Aims: CYP1A1 and CYP1B1, members of the cytochrome P450 protein family, are regulated by fluid shear stress. This study describes the effects of duration, magnitude and pattern of shear stress on CYP1A1 and CYP1B1 expressions in human endothelial cells, towards the goal of understanding the role(s) of these genes in pro-atherogenic or anti-atherogenic endothelial cell functions.
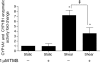
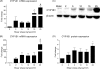
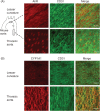
Methods and results: We investigated CYP1A1 and CYP1B1 expressions under different durations, levels, and patterns of shear stress. CYP1A1 and CYP1B1 mRNA, protein, and enzymatic activity were maximally up-regulated at > or =24 h of arterial levels of shear stress (15-25 dynes/cm2). Expression of both genes was significantly attenuated by reversing shear stress when compared with 15 dynes/cm2 steady shear stress. Small interfering RNA knockdown of CYP1A1 resulted in significantly reduced CYP1B1 and thrombospondin-1 expression, genes regulated by the aryl hydrocarbon receptor (AhR). Immunostaining of human coronary arteries showed constitutive CYP1A1 and CYP1B1 protein expressions in endothelial cells. Immunostaining of mouse aorta showed nuclear localization of AhR and increased expression of CYP1A1 in the descending thoracic aorta, whereas reduced nuclear localization of AhR and attenuated CYP1A1 expression were observed in the lesser curvature of the aortic arch.
Conclusion: CYP1A1 and CYP1B1 gene and protein expressions vary with time, magnitude, and pattern of shear stress. Increased CYP1A1 gene expression modulates AhR-regulated genes. Based on our in vitro reversing flow data and in vivo immunostained mouse aorta, we suggest that increased expression of both genes reflects an anti-atherogenic endothelial cell phenotype.
Figures
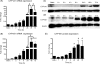
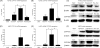
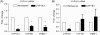
Comment in
-
Cytochromes CYP1A1 and CYP1B1: new pieces in the puzzle to understand the biomechanical paradigm of atherosclerosis.Cardiovasc Res. 2009 Mar 1;81(4):629-32. doi: 10.1093/cvr/cvp013. Epub 2009 Jan 15. Cardiovasc Res. 2009. PMID: 19147650 No abstract available.
References
-
- Wootton DM, Ku DN. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu Rev Biomed Eng. 1999;1:299–329. - PubMed
-
- Davies PF. Endothelial transcriptome profiles in vivo in complex arterial flow fields. Ann Biomed Eng. 2008;36:563–570. - PubMed
-
- Michaelis UR, Fleming I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol Ther. 2006;111:584–595. - PubMed
-
- Barouki R, Morel Y. Repression of cytochrome P450 1A1 gene expression by oxidative stress: mechanisms and biological implications. Biochem Pharmacol. 2001;61:511–516. - PubMed

