Elevated NCOR1 disrupts a network of dietary-sensing nuclear receptors in bladder cancer cells
- PMID: 19126649
- PMCID: PMC2722152
- DOI: 10.1093/carcin/bgp005
Elevated NCOR1 disrupts a network of dietary-sensing nuclear receptors in bladder cancer cells
Abstract
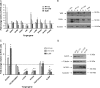
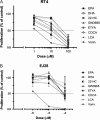
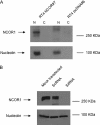
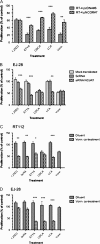
Increasingly invasive bladder cancer cells lines displayed insensitivity toward a panel of dietary-derived ligands for members of the nuclear receptor superfamily. Insensitivity was defined through altered gene regulatory actions and cell proliferation and reflected both reduced receptor expression and elevated nuclear receptor corepressor 1 (NCOR1) expression. Stable overexpression of NCOR1 in sensitive cells (RT4) resulted in a panel of clones that recapitulated the resistant phenotype in terms of gene regulatory actions and proliferative responses toward ligand. Similarly, silencing RNA approaches to NCOR1 in resistant cells (EJ28) enhanced ligand gene regulatory and proliferation responses, including those mediated by peroxisome proliferator-activated receptor (PPAR) gamma and vitamin D receptor (VDR) receptors. Elevated NCOR1 levels generate an epigenetic lesion to target in resistant cells using the histone deacetylase inhibitor vorinostat, in combination with nuclear receptor ligands. Such treatments revealed strong-additive interactions toward the PPARgamma, VDR and Farnesoid X-activated receptors. Genome-wide microarray and microfluidic quantitative real-time, reverse transcription-polymerase chain reaction approaches, following the targeting of NCOR1 activity and expression, revealed the selective capacity of this corepressor to govern common transcriptional events of underlying networks. Combined these findings suggest that NCOR1 is a selective regulator of nuclear receptors, notably PPARgamma and VDR, and contributes to their loss of sensitivity. Combinations of epigenetic therapies that target NCOR1 may prove effective, even when receptor expression is reduced.
Figures

References
-
- Crescioli C, et al. Human bladder as a novel target for vitamin D receptor ligands. J. Clin. Endocrinol. Metab. 2005;90:962–972. - PubMed
-
- Shen SS, et al. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer. 2006;106:2610–2616. - PubMed
-
- Kassouf W, et al. Inhibition of bladder tumor growth by 1,1-bis(3′-indolyl)-1-(p-substitutedphenyl)methanes: a new class of peroxisome proliferator-activated receptor gamma agonists. Cancer Res. 2006;66:412–418. - PubMed
-
- Makishima M, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. - PubMed
-
- Shaw N, et al. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J. Biol. Chem. 2003;278:41589–41592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

