Connexin mutation that causes dominant congenital cataracts inhibits gap junctions, but not hemichannels, in a dominant negative manner
- PMID: 19126675
- PMCID: PMC2650834
- DOI: 10.1242/jcs.034124
Connexin mutation that causes dominant congenital cataracts inhibits gap junctions, but not hemichannels, in a dominant negative manner
Abstract
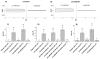
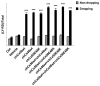
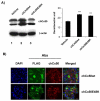
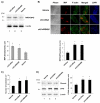
The connexin (Cx) 50, E48K, mutation is associated with a human dominant congenital cataract; however, the underlying molecular mechanism has not been characterized. The glutamate (E) residue at position 48 is highly conserved across animal species and types of connexins. When expressed in paired Xenopus oocytes, human (h) and chicken (ch) Cx50 E48K mutants showed no electrical coupling. In addition, this mutation acts in a dominant negative manner when paired hetero-typically or hetero-merically with wild-type Cx50, but has no such effect on Cx46, the other lens fiber connexin. A similar loss-of-function and dominant negative effect was observed using dye transfer assays in the same system. By using two different dye transfer methods, with two different tracer dyes, we found chCx50 E48K expressed in chicken lens embryonic fibroblast cells by retroviral infection similarly failed to induce dye coupling, and prevented wild-type chCx50 from forming functional gap junctions. In contrast to its effect on gap junctions, the E48K mutation has no effect on hemichannel activity when assayed using electrical conductance in oocytes, and mechanically induced dye uptake in cells. Cx50 is functionally involved in cell differentiation and lens development, and the E48K mutant promotes primary lens cell differentiation indistinguishable from wild-type chCx50, despite its lack of junctional channel function. Together the data show that mutations affecting gap junctions but not hemichannel function of Cx50 can lead to dominant congenital cataracts in humans. This clearly supports the model of intercellular coupling of fiber cells creating a microcirculation of nutrients and metabolites required for lens transparency.
Figures
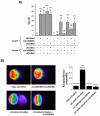
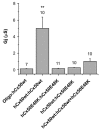

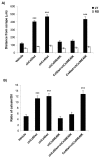
References
-
- Banks, E. A., Yu, X. S., Shi, Q. and Jiang, J. X. (2007). Promotion of lens epithelial-fiber differentiation by the C-terminus of connexin 45.6 a role independent of gap junction communication. J. Cell Sci. 120, 3602-3612. - PubMed
-
- Bao, L., Sachs, F. and Dahl, G. (2005). Connexins are mechanosensitive. Am. J. Physiol., Cell Physiol. 287 C1389-C1395. - PubMed
-
- Berry, V., Mackay, D., Khaliq, S., Francis, P. J., Hameed, A., Anwar, K., Mehdi, S. Q., Newbold, R. J., Ionides, A., Shiels, A. et al. (1999). Connexin 50 mutation in a family with congenital “zonular nuclear” pulverulent cataract of Pakistani origin. Hum. Genet. 105, 168-170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

