Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy
- PMID: 19126686
- PMCID: PMC2626743
- DOI: 10.1073/pnas.0811022106
Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy
Abstract
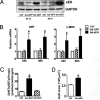
Pathophysiological cardiac hypertrophy is one of the most common causes of heart failure. Epoxyeicosatrienoic acids, hydrolyzed and degraded by soluble epoxide hydrolase (sEH), can function as endothelium-derived hyperpolarizing factors to induce dilation of coronary arteries and thus are cardioprotective. In this study, we investigated the role of sEH in two rodent models of angiotensin II (Ang II)-induced cardiac hypertrophy. The protein level of sEH was elevated in the heart of both spontaneously hypertensive rats and Ang II-infused Wistar rats. Blocking the Ang II type 1 receptor with losartan could abolish this induction. Administration of a potent sEH inhibitor (sEHI) prevented the pathogenesis of the Ang II-induced hypertrophy, as demonstrated by decreased left-ventricular hypertrophy assessed by echocardiography, reduced cardiomyocyte size, and attenuated expression of hypertrophy markers, including atrial natriuretic factor and beta-myosin heavy chain. Because sEH elevation was not observed in exercise- or norepinephrine-induced hypertrophy, the sEH induction was closely associated with Ang II-induced hypertrophy. In vitro, Ang II upregulated sEH and hypertrophy markers in neonatal cardiomyocytes isolated from rat and mouse. Expression of these marker genes was elevated with adenovirus-mediated sEH overexpression but decreased with sEHI treatment. These results were supported by studies in neonatal cardiomyocytes from sEH(-/-) mice. Our results suggest that sEH is specifically upregulated by Ang II, which directly mediates Ang II-induced cardiac hypertrophy. Thus, pharmacological inhibition of sEH would be a useful approach to prevent and treat Ang II-induced cardiac hypertrophy.
Conflict of interest statement
Conflict of interest statement: P.D.J., N.C., and B.D.H. authored University of California patents in this area. B. D. Hammock founded Arete Therapeutics to evaulate inhibitors of the soluble epoxide hydrolase as therapeutic agents. Arete has licensed UC IP in this area. None of the authors received funding for this work from Arete Therepeutics, and Arete Therapeutics scientists did not contribute to these studies.
Figures
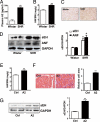
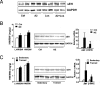
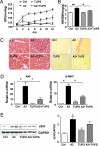
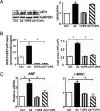
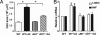
References
-
- Katz AM. The cardiomyopathy of overload: an unnatural growth response. Eur Heart J. 1995;16(Suppl O):110–114. - PubMed
-
- Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17(16):1937–1956. - PubMed
-
- Morkin E. Regulation of myosin heavy chain genes in the heart. Circulation. 1993;87(5):1451–1460. - PubMed
-
- de Bold AJ, et al. The physiological and pathophysiological modulation of the endocrine function of the heart. Can J Physiol Pharmacol. 2001;79(8):705–714. - PubMed
-
- Mann DL. Heart Failure: A Companion to Braunwald's Heart Disease. Philadelphia: Saunders; 2004. pp. 10–40.
Publication types
MeSH terms
Substances
Grants and funding
- HL 85727/HL/NHLBI NIH HHS/United States
- F32 ES005707/ES/NIEHS NIH HHS/United States
- R01 HL085727/HL/NHLBI NIH HHS/United States
- R01 ES002710/ES/NIEHS NIH HHS/United States
- P30 ES005707/ES/NIEHS NIH HHS/United States
- P42 ES004699/ES/NIEHS NIH HHS/United States
- P42/PHS HHS/United States
- ES04699/ES/NIEHS NIH HHS/United States
- R37 ES02710/ES/NIEHS NIH HHS/United States
- F32 HL078096/HL/NHLBI NIH HHS/United States
- R37 ES002710/ES/NIEHS NIH HHS/United States
- P42 ES05707/ES/NIEHS NIH HHS/United States
- P42 ES04699/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

