Estrogen replacement enhances EDHF-mediated vasodilation of mesenteric and uterine resistance arteries: role of endothelial cell Ca2+
- PMID: 19126786
- PMCID: PMC2660142
- DOI: 10.1152/ajpendo.90517.2008
Estrogen replacement enhances EDHF-mediated vasodilation of mesenteric and uterine resistance arteries: role of endothelial cell Ca2+
Abstract
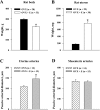
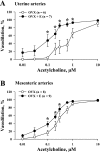
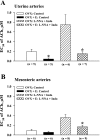
Endothelium-derived hyperpolarizing factor (EDHF) plays an important role in the regulation of vascular microcirculatory tone. This study explores the role of estrogen in controlling EDHF-mediated vasodilation of uterine resistance arteries of the rat and also analyzes the contribution of endothelial cell (EC) Ca(2+) signaling to this process. A parallel study was also performed with mesenteric arteries to provide comparison with a nonreproductive vasculature. Mature female rats underwent ovariectomy, with one half receiving 17beta-estradiol replacement (OVX+E) and the other half serving as estrogen-deficient controls (OVX). Uterine or mesenteric resistance arteries were harvested, cannulated, and pressurized. Nitric oxide and prostacyclin production were inhibited with 200 microM N(G)-nitro-l-arginine and 10 microM indomethacin, respectively. ACh effectively dilated the arteries preconstricted with phenylephrine but failed to induce dilation of vessels preconstricted with high-K(+) solution. ACh EC(50) values were decreased by estrogen replacement by five- and twofold in uterine and mesenteric arteries, respectively. As evidenced by fura-2-based measurements of EC cytoplasmic Ca(2+) concentration ([Ca(2+)](i)), estrogen replacement was associated with increased basal and ACh-stimulated EC [Ca(2+)](i) rise in uterine, but not mesenteric, vessels. These data demonstrate that EDHF contributes to endothelium-dependent vasodilation of uterine and mesenteric resistance arteries and that estrogen controls EDHF-related mechanism(s) more efficiently in reproductive vs. nonreproductive vessels. Enhanced endothelial Ca(2+) signaling may be an important underlying mechanism in estrogenic modulation of EDHF-mediated vasodilation in small resistance uterine arteries.
Figures





References
-
- Abdalla FM, Marostica E, Picarelli ZP, Abreu LC, Avellar MC, Porto CS. Effect of estrogen on muscarinic acetylcholine receptor expression in rat myometrium. Mol Cell Endocrinol 213: 139–148, 2004. - PubMed
-
- Adeagbo AS, Triggle CR. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J Cardiovasc Pharmacol 21: 423–429, 1993. - PubMed
-
- Anari MR, Bakhtiar R, Zhu B, Huskey S, Franklin RB, Evans DC. Derivatization of ethinylestradiol with dansyl chloride to enhance electrospray ionization: application in trace analysis of ethinylestradiol in rhesus monkey plasma. Anal Chem 74: 4136–4144, 2002. - PubMed
-
- Bosch MA, Kelly MJ, Ronnekleiv OK. Distribution, neuronal colocalization, and 17beta-E2 modulation of small conductance calcium-activated K+ channel (SK3) mRNA in the guinea pig brain. Endocrinology 143: 1097–1107, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

