Oxidative imbalance in nonstimulated X-adrenoleukodystrophy-derived lymphoblasts
- PMID: 19127062
- PMCID: PMC2744110
- DOI: 10.1159/000191212
Oxidative imbalance in nonstimulated X-adrenoleukodystrophy-derived lymphoblasts
Abstract
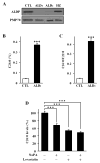
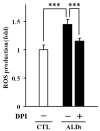
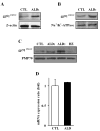
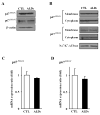
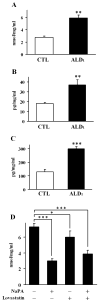
X-Adrenoleukodystrophy (X-ALD) is a peroxisomal disorder characterized by accumulation of very-long-chain (VLC) fatty acids, which induces inflammatory disease and alterations in cellular redox, both of which are reported to play a role in the pathogenesis of the severe form of the disease (childhood cerebral ALD). Here, we report on the status of oxidative stress (NADPH oxidase activity) and inflammatory mediators in an X-ALD lymphoblast cell line under nonstimulated conditions. X-ALD lymphoblasts contain nearly 7 times higher levels of the C(26:0) fatty acid compared to controls; these levels were downregulated by treatment with sodium phenylacetate (NaPA), lovastatin or the combination of both drugs. In addition, free-radicals synthesis was elevated in X-ALD lymphoblasts, and protein levels of the NADPH oxidase gp91(PHOX) membrane subunit were significantly upregulated, but no changes were observed in the p47(PHOX) and p67(PHOX) cytoplasmic subunits. Unexpectedly, there was no increase in gp91(PHOX) mRNA levels in X-ALD lymphoblasts. Furthermore, X-ALD lymphoblasts produced higher levels of nitric oxide (NO) and cytokines (tumor necrosis factor-alpha and interleukin 1 beta), and treatment with NaPA or lovastatin decreased the synthesis of NO. Our data indicate that X-ALD lymphoblasts are significantly affected by the accumulation of VLC fatty acids, which induces changes in the cell membrane properties/functions that may, in turn, play a role in the development/progression of the pathogenesis of X-ALD disease.
(c) 2009 S. Karger AG, Basel.
Figures
References
-
- Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. 2006;75:295–332. - PubMed
-
- Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, Poustka AM, et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. - PubMed
-
- Contreras M, Mosser J, Mandel JL, Aubourg P, Singh I. The protein coded by the X-adrenoleukodystrophy gene is a peroxisomal integral membrane protein. FEBS Lett. 1994;344:211–215. - PubMed
-
- Contreras M, Sengupta TK, Sheikh F, Aubourg P, Singh I. Topology of ATP-binding domain of adrenoleukodystrophy gene product in peroxisomes. Arch Biochem Biophys. 1996;334:369–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

