Indinavir-loaded pH-sensitive microparticles for taste masking: toward extemporaneous pediatric anti-HIV/AIDS liquid formulations with improved patient compliance
- PMID: 19127436
- PMCID: PMC2663661
- DOI: 10.1208/s12249-008-9168-z
Indinavir-loaded pH-sensitive microparticles for taste masking: toward extemporaneous pediatric anti-HIV/AIDS liquid formulations with improved patient compliance
Abstract
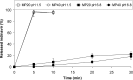
The aim of this work was to develop indinavir pediatric anti-HIV/AIDS formulations enabling convenient dose adjustment, ease of oral administration, and improved organoleptic properties by means of the generation of drug-loaded microparticles made of a polymer that is insoluble under intake conditions and dissolves fast in the stomach in order to completely release the active agent. Indinavir-loaded microparticles made of a pH-dependent polymeric excipient soluble at pH < 5, Eudragit E100, were prepared using a double emulsion solvent diffusion technique and the in vitro release profiles characterized. Finally, taste masking properties were evaluated in blind randomized sensory experiments by ten healthy human volunteers. The use of a w/o/o emulsion system resulted in indinavir loads around 90%. Thermal analysis of the microparticles by differential scanning calorimetry revealed that indinavir appeared mainly dispersed at the molecular level. Concentrations of residual organic solvents as determined by gas chromatography were below the upper limits specified by the European Pharmacopeia for pharmaceutical oral formulations. Then, the behavior of drug-containing microparticles in aqueous media at different pH values was assessed. While they selectively dissolved in gastric-like medium, in tap water (intake conditions), the matrix remained almost unchanged and efficiently prevented drug dissolution. Finally, sensoring taste tests performed by volunteers indicated that systems with indinavir loads approximately 15% displayed acceptable taste. This work explored the production of indinavir-containing microparticles based on a common pharmaceutical excipient as a means for the improvement of medicines of drugs involved in the treatment of HIV/AIDS. For systems containing about 15% drug, taste studies confirmed the acceptability of the formulation. In pediatric regimes, this composition would require an acceptable amount of formulation (0.7-1.5 g).
Figures



References
-
- Van Dyke R.B., Lee S., Johnson G.M., Wiznia A., Mohan K., Stanley K., Morse E.V., Krogstad P.A., Nachman S. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. 2002;109:61–67. doi: 10.1542/peds.109.4.e61. - DOI - PubMed
-
- WHO/Make medicines child size, In http://www.who.int/childmedicines/en/ (accessed 01/07/08).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

