Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes
- PMID: 19127517
- PMCID: PMC2753874
- DOI: 10.1002/hep.22664
Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes
Abstract
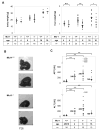
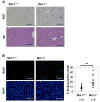
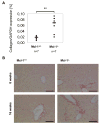
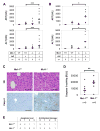
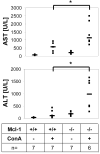
Myeloid cell leukemia-1 (Mcl-1) is an antiapoptotic member of the Bcl-2 protein family. It interacts with proapoptotic Bcl-2 family members, thereby inhibiting mitochondrial activation and induction of apoptosis. Mcl-1 is essential for embryonal development and the maintenance of B cells, T cells, and hematopoietic stem cells. We have recently shown that induction of Mcl-1 by growth factors rescues primary human hepatocytes from CD95-mediated apoptosis. This prompted us to further analyze the relevance of Mcl-1 for hepatocellular homeostasis. Therefore, we generated a hepatocyte-specific Mcl-1 knockout mouse (Mcl-1(flox/flox)-AlbCre). Deletion of Mcl-1 in hepatocytes results in liver cell damage caused by spontaneous induction of apoptosis. Livers of Mcl-1(flox/flox)-AlbCre mice are smaller compared to control littermates, due to higher apoptosis rates. As a compensatory mechanism, proliferation of hepatocytes is enhanced in the absence of Mcl-1. Importantly, hepatic pericellular fibrosis occurs in Mcl-1 negative livers in response to chronic liver damage. Furthermore, Mcl-1(flox/flox)-AlbCre mice are more susceptible to hepatocellular damage induced by agonistic anti-CD95 antibodies or concanavalin A.
Conclusion: The present study provides in vivo evidence that Mcl-1 is a crucial antiapoptotic factor for the liver, contributing to hepatocellular homeostasis and protecting hepatocytes from apoptosis induction.
Figures


References
-
- Baskin-Bey ES, Gores GJ. Death by association: BH3 domain-only proteins and liver injury. Am J Physiol Gastrointest Liver Physiol. 2005;289:G987–990. - PubMed
-
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. - PubMed
-
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
