Single myosin lever arm orientation in a muscle fiber detected with photoactivatable GFP
- PMID: 19127992
- PMCID: PMC2709297
- DOI: 10.1021/bi8017703
Single myosin lever arm orientation in a muscle fiber detected with photoactivatable GFP
Abstract
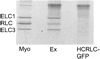
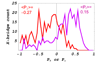
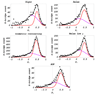
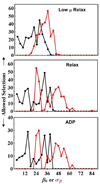
Myosin 2 is the molecular motor in muscle. It binds actin and executes a power stroke by rotating its lever arm through an angle of approximately 70 degrees to translate actin against resistive force. Myosin 2 has evolved to function optimally under crowded conditions where rates and equilibria of macromolecular reactions undergo major shifts relative to those measured in dilute solution. Hence, an important research objective is to detect in situ the lever arm orientation. Single-molecule measurements are preferred because they clarify ambiguities that are unavoidable with ensemble measurements; however, detecting single molecules in the condensed tissue medium where the myosin concentration exceeds 100 muM is challenging. A myosin light chain (MLC) tagged with photoactivatable green fluorescent protein (PAGFP) was constructed. The recombinant MLC physically and functionally replaced native MLC on the myosin lever arm in a permeabilized skeletal muscle fiber. Probe illumination volume was minimized using total internal reflection fluorescence microscopy, and PAGFP was sparsely photoactivated such that polarized fluorescence identified a single probe orientation. Several physiological states of the muscle fiber were characterized, revealing two distinct orientation populations in all states called straight and bent conformations. Conformation occupancy probability varies among fiber states with rigor and isometric contraction at extremes where straight and bent conformations predominate, respectively. Comparison to previous work on single rigor cross-bridges at the A-band periphery where the myosin concentration is low suggests molecular crowding in the A-band promotes occupancy of the straight myosin conformation [Burghardt, T. P., et al. (2007) Biophys. J. 93, 2226]. The latter may have a role in contraction because it provides additional free energy favoring completion of the cross-bridge power stroke.
Figures







References
-
- Huxley HE. The mechanism of muscular contraction. Science. 1969;164:1356–1366. - PubMed
-
- Peyser YM, Ajtai K, Werber MM, Burghardt TP, Muhlrad A. Effect of metal cations on the conformation of myosin subfragment-1-ADP-phosphate analog complexes: A near UV circular dichroism study. Biochemistry. 1997;36:5170–5178. - PubMed
-
- Trentham DR, Eccleston JF, Bagshaw CR. Kinetic analysis of ATPase mechanisms. Quarterly Reviews of Biophysics. 1976;9:217–281. - PubMed
-
- Marston SB, Taylor EW. Comparison of the myosin and actomyosin ATPase mechanisms of the four types of vertebrate muscles. J. Mol. Biol. 1980;139:573–600. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

