Mechanism of interferon-gamma-induced increase in T84 intestinal epithelial tight junction
- PMID: 19128033
- PMCID: PMC2988462
- DOI: 10.1089/jir.2008.0128
Mechanism of interferon-gamma-induced increase in T84 intestinal epithelial tight junction
Abstract
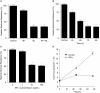
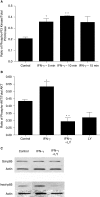
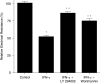
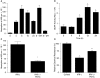
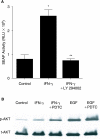
Interferon-gamma (IFN-gamma) is an important proinflammatory cytokine that plays a central role in the intestinal inflammatory process of inflammatory bowel disease. IFN-gamma induced disturbance of the intestinal epithelial tight junction (TJ) barrier has been postulated to be an important mechanism contributing to intestinal inflammation. The intracellular mechanisms that mediate the IFN-gamma induced increase in intestinal TJ permeability remain unclear. The aim of this study was to examine the role of the phosphatidylinositol 3-kinase (PI3-K) pathway in the regulation of the IFN-gamma induced increase in intestinal TJ permeability using the T84 intestinal epithelial cell line. IFN-gamma caused an increase in T84 intestinal epithelial TJ permeability and depletion of TJ protein, occludin. The IFN-gamma induced increase in TJ permeability and alteration in occludin protein was associated with rapid activation of PI3-K; and inhibition of PI3-K activation prevented the IFN-gamma induced effects. IFN-gamma also caused a delayed but more prolonged activation of nuclear factor-kappaB (NF-kappaB); inhibition of NF-kappaB also prevented the increase in T84 TJ permeability and alteration in occludin expression. The IFN-gamma induced activation of NF-kappaB was mediated by a cross-talk with PI3-K pathway. In conclusion, the IFN-gamma induced increase in T84 TJ permeability and alteration in occludin protein expression were mediated by the PI3-K pathway. These results show for the first time that the IFN-gamma modulation of TJ protein and TJ barrier function is regulated by a cross-talk between PI3-K and NF-kappaB pathways.
Figures

References
-
- Adams RB. Planchon SM. Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–2363. - PubMed
-
- Bruewer M. Luegering A. Kucharzik T. Parkos CA. Madara JL. Hopkins AM. Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

