Enantioselective, ketoreductase-based entry into pharmaceutical building blocks: ethanol as tunable nicotinamide reductant
- PMID: 19128188
- PMCID: PMC6027600
- DOI: 10.1021/ol802464g
Enantioselective, ketoreductase-based entry into pharmaceutical building blocks: ethanol as tunable nicotinamide reductant
Abstract
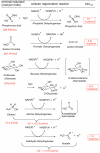
The use of NADH- and NADPH-dependent ketoreductases to access enantioenriched pharmaceutical building blocks is reported. Seven structurally diverse synthons are obtained, including those for atomoxetine (KRED 132), talampanel (RS1-ADH and CPADH), Dolastatin (KRED 132), and fluoxetine (KRED 108/132). Ethanol may be used as stoichiometric reductant, regenerating both nicotinamide cofactors, particularly under four-electron redox conditions. Its favorable thermodynamic and economic profile, coupled with its advantageous dual cosolvent role, suggests a new application for biomass-derived ethanol.
Figures

Similar articles
-
A strategy to identify a ketoreductase that preferentially synthesizes pharmaceutically relevant (S)-alcohols using whole-cell biotransformation.Microb Cell Fact. 2018 Dec 3;17(1):192. doi: 10.1186/s12934-018-1036-2. Microb Cell Fact. 2018. PMID: 30509260 Free PMC article.
-
In vitro co-metabolism of ethanol and cyclic ketones.Toxicology. 2002 Aug 15;177(2-3):207-13. doi: 10.1016/s0300-483x(02)00221-4. Toxicology. 2002. PMID: 12135624
-
Towards catalyst compartimentation in combined chemo- and biocatalytic processes: immobilization of alcohol dehydrogenases for the diastereoselective reduction of a β-hydroxy ketone obtained from an organocatalytic aldol reaction.J Biotechnol. 2013 Nov;168(3):271-6. doi: 10.1016/j.jbiotec.2013.08.031. Epub 2013 Sep 11. J Biotechnol. 2013. PMID: 24036136
-
Impact and relevance of alcohol dehydrogenase enantioselectivities on biotechnological applications.Appl Microbiol Biotechnol. 2020 Apr;104(7):2897-2909. doi: 10.1007/s00253-020-10440-2. Epub 2020 Feb 15. Appl Microbiol Biotechnol. 2020. PMID: 32060695 Review.
-
Ethanol cycle in an ethanologenic bacterium.FEBS Lett. 2002 Jul 3;522(1-3):6-8. doi: 10.1016/s0014-5793(02)02923-x. FEBS Lett. 2002. PMID: 12095609 Review.
Cited by
-
Biomacromolecule-Assisted Screening for Reaction Discovery and Catalyst Optimization.Chem Rev. 2022 Aug 24;122(16):13800-13880. doi: 10.1021/acs.chemrev.2c00213. Epub 2022 Jul 29. Chem Rev. 2022. PMID: 35904776 Free PMC article. Review.
-
Combinatorial catalysis employing a visible enzymatic beacon in real time: synthetically versatile (pseudo)halometalation/carbocyclizations.Angew Chem Int Ed Engl. 2011 Sep 12;50(38):8895-9. doi: 10.1002/anie.201103365. Epub 2011 Aug 16. Angew Chem Int Ed Engl. 2011. PMID: 21905180 Free PMC article. No abstract available.
-
Combining a Clostridial enzyme exhibiting unusual active site plasticity with a remarkably facile sigmatropic rearrangement: rapid, stereocontrolled entry into densely functionalized fluorinated phosphonates for chemical biology.J Am Chem Soc. 2015 Mar 18;137(10):3600-9. doi: 10.1021/jacs.5b00022. Epub 2015 Mar 5. J Am Chem Soc. 2015. PMID: 25719907 Free PMC article.
-
Use of a robust dehydrogenase from an archael hyperthermophile in asymmetric catalysis-dynamic reductive kinetic resolution entry into (S)-profens.J Am Chem Soc. 2010 May 5;132(17):5930-1. doi: 10.1021/ja910778p. J Am Chem Soc. 2010. PMID: 20377222 Free PMC article.
-
Is a Malleable Active Site Loop the Key to High Substrate Promiscuity? Hybrid, Biocatalytic Route to Structurally Diverse Taxoid Side Chains with Remarkable Dual Stereocontrol.Angew Chem Int Ed Engl. 2025 Sep 1;64(36):e202510889. doi: 10.1002/anie.202510889. Epub 2025 Jun 30. Angew Chem Int Ed Engl. 2025. PMID: 40537411 Free PMC article.
References
-
- Moore JC Pollard DJ Kosjek B Devine PN Acc. Chem. Res 2007. 40 1412–1419 - PubMed
-
- Kaluzna IA Feske BD Wittayanan W Ghiviriga I Stewart JD J. Org. Chem 2005. 70 342–345 - PubMed
-
- Berkowitz DB Choi S Maeng J-H J. Org. Chem 2000. 65 847–860 - PubMed
- Berkowitz DB Hartung RE Choi S Tetrahedron: Asymmetry 1999. 10 4513–4520
- Berkowitz DB Maeng J-H Dantzig AH Shepard RL Norman BH J. Am. Chem. Soc 1996. 118 9426–9427
-
- Berkowitz DB Pumphrey JA Shen Q Tetrahedron Lett 1994. 35 8743–8746
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

