Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila
- PMID: 19128239
- PMCID: PMC2842572
- DOI: 10.1042/BJ20082003
Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila
Abstract
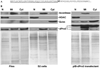
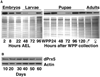
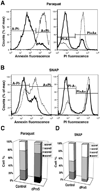
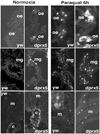
Peroxiredoxin 5 is a distinct isoform of the peroxiredoxin gene family. The antioxidative and anti-apoptotic functions of peroxiredoxin 5 have been extensively demonstrated in cell culture experiments. In the present paper, we provide the first functional analysis of peroxiredoxin 5 in a multicellular organism, Drosophila melanogaster. Similar to its mammalian, yeast or human counterparts, dPrx5 (Drosophila peroxiredoxin 5) is expressed in several cellular compartments, including the cytosol, nucleus and the mitochondrion. Global overexpression of dPrx5 in flies increased resistance to oxidative stress and extended their life span by up to 30% under normal conditions. The dprx5(-/-) null flies were comparatively more susceptible to oxidative stress, had higher incidence of apoptosis, and a shortened life span. TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling) analysis revealed that the dprx5(-/-) null mutant had discernible tissue-specific apoptotic patterns, similar to those observed in control flies exposed to paraquat. In addition, apoptosis was particularly notable in oenocytes. During development the dPrx5 levels co-varied with ecdysone pulses, suggesting inter-relationship between ecdystreroids and dPrx5 expression. The importance of dPrx5 for development was further underscored by the embryonic lethal phenotype of progeny derived from the dprx5(-/-) null mutant. Results from the present study suggest that the antioxidant and anti-apoptotic activities of dPrx5 play a critical role in development and aging of the fly.
Figures



References
-
- Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J. Biol. Chem. 2003;278:25179–25190. - PubMed
-
- Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Peroxiredoxin 6 gene-targeted mice show increased lung injury with paraquat-induced oxidative stress. Antioxid. Redox Signaling. 2006;8:229–237. - PubMed
-
- Nagy N, Malik G, Fisher AB, Das DK. Targeted disruption of peroxiredoxin 6 gene renders the heart vulnerable to ischemia-reperfusion injury. Am. J. Physiol. 2006;291:H2636–H2640. - PubMed
-
- Li L, Shoji W, Oshima H, Obinata M, Fukumoto M, Kanno N. Crucial role of peroxiredoxin III in placental antioxidant defense of mice. FEBS Lett. 2008;582:2431–2434. - PubMed
-
- Egler RA, Fernandes E, Rothermund K, Sereika S, de Souza-Pinto N, Jaruga P, Dizdaroglu M, Prochownik EV. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24:8038–8050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

