Reporter gene-expressing bone marrow-derived stromal cells are immune-tolerated following implantation in the central nervous system of syngeneic immunocompetent mice
- PMID: 19128466
- PMCID: PMC2630974
- DOI: 10.1186/1472-6750-9-1
Reporter gene-expressing bone marrow-derived stromal cells are immune-tolerated following implantation in the central nervous system of syngeneic immunocompetent mice
Abstract
Background: Cell transplantation is likely to become an important therapeutic tool for the treatment of various traumatic and ischemic injuries to the central nervous system (CNS). However, in many pre-clinical cell therapy studies, reporter gene-assisted imaging of cellular implants in the CNS and potential reporter gene and/or cell-based immunogenicity, still remain challenging research topics.
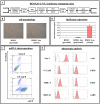
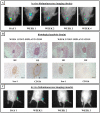
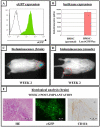
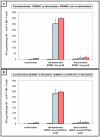
Results: In this study, we performed cell implantation experiments in the CNS of immunocompetent mice using autologous (syngeneic) luciferase-expressing bone marrow-derived stromal cells (BMSC-Luc) cultured from ROSA26-L-S-L-Luciferase transgenic mice, and BMSC-Luc genetically modified using a lentivirus encoding the enhanced green fluorescence protein (eGFP) and the puromycin resistance gene (Pac) (BMSC-Luc/eGFP/Pac). Both reporter gene-modified BMSC populations displayed high engraftment capacity in the CNS of immunocompetent mice, despite potential immunogenicity of introduced reporter proteins, as demonstrated by real-time bioluminescence imaging (BLI) and histological analysis at different time-points post-implantation. In contrast, both BMSC-Luc and BMSC-Luc/eGFP/Pac did not survive upon intramuscular cell implantation, as demonstrated by real-time BLI at different time-points post-implantation. In addition, ELISPOT analysis demonstrated the induction of IFN-gamma-producing CD8+ T-cells upon intramuscular cell implantation, but not upon intracerebral cell implantation, indicating that BMSC-Luc and BMSC-Luc/eGFP/Pac are immune-tolerated in the CNS. However, in our experimental transplantation model, results also indicated that reporter gene-specific immune-reactive T-cell responses were not the main contributors to the immunological rejection of BMSC-Luc or BMSC-Luc/eGFP/Pac upon intramuscular cell implantation.
Conclusion: We here demonstrate that reporter gene-modified BMSC derived from ROSA26-L-S-L-Luciferase transgenic mice are immune-tolerated upon implantation in the CNS of syngeneic immunocompetent mice, providing a research model for studying survival and localisation of autologous BMSC implants in the CNS by real-time BLI and/or histological analysis in the absence of immunosuppressive therapy.
Figures
References
-
- Li J, Sun CR, Zhang H, Tsang KS, Li JH, Zhang SD, An YH. Induction of functional recovery by co-transplantation of neural stem cells and Schwann cells in a rat spinal cord contusion injury model. Biomed Environ Sci. 2007;20:242–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

