Flux balance analysis of primary metabolism in Chlamydomonas reinhardtii
- PMID: 19128495
- PMCID: PMC2628641
- DOI: 10.1186/1752-0509-3-4
Flux balance analysis of primary metabolism in Chlamydomonas reinhardtii
Abstract
Background: Photosynthetic organisms convert atmospheric carbon dioxide into numerous metabolites along the pathways to make new biomass. Aquatic photosynthetic organisms, which fix almost half of global inorganic carbon, have great potential: as a carbon dioxide fixation method, for the economical production of chemicals, or as a source for lipids and starch which can then be converted to biofuels. To harness this potential through metabolic engineering and to maximize production, a more thorough understanding of photosynthetic metabolism must first be achieved. A model algal species, C. reinhardtii, was chosen and the metabolic network reconstructed. Intracellular fluxes were then calculated using flux balance analysis (FBA).
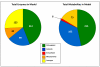
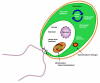
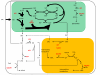
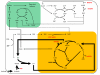
Results: The metabolic network of primary metabolism for a green alga, C. reinhardtii, was reconstructed using genomic and biochemical information. The reconstructed network accounts for the intracellular localization of enzymes to three compartments and includes 484 metabolic reactions and 458 intracellular metabolites. Based on BLAST searches, one newly annotated enzyme (fructose-1,6-bisphosphatase) was added to the Chlamydomonas reinhardtii database. FBA was used to predict metabolic fluxes under three growth conditions, autotrophic, heterotrophic and mixotrophic growth. Biomass yields ranged from 28.9 g per mole C for autotrophic growth to 15 g per mole C for heterotrophic growth.
Conclusion: The flux balance analysis model of central and intermediary metabolism in C. reinhardtii is the first such model for algae and the first model to include three metabolically active compartments. In addition to providing estimates of intracellular fluxes, metabolic reconstruction and modelling efforts also provide a comprehensive method for annotation of genome databases. As a result of our reconstruction, one new enzyme was annotated in the database and several others were found to be missing; implying new pathways or non-conserved enzymes. The use of FBA to estimate intracellular fluxes also provides flux values that can be used as a starting point for rational engineering of C. reinhardtii. From these initial estimates, it is clear that aerobic heterotrophic growth on acetate has a low yield on carbon, while mixotrophically and autotrophically grown cells are significantly more carbon efficient.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

