Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice
- PMID: 19128794
- PMCID: PMC2713676
- DOI: 10.1016/j.stem.2008.10.015
Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice
Abstract
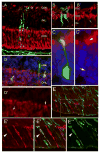
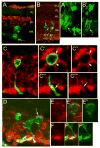
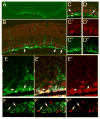
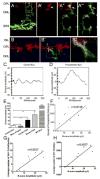
Some of the most common causes of blindness involve the degeneration of photoreceptors in the neural retina; photoreceptor replacement therapy might restore some vision in these individuals. Embryonic stem cells (ESCs) could, in principle, provide a source of photoreceptors to repair the retina. We have previously shown that retinal progenitors can be efficiently derived from human ESCs. We now show that retinal cells derived from human ESCs will migrate into mouse retinas following intraocular injection, settle into the appropriate layers, and express markers for differentiated cells, including both rod and cone photoreceptor cells. After transplantation of the cells into the subretinal space of adult Crx(-/-) mice (a model of Leber's Congenital Amaurosis), the hESC-derived retinal cells differentiate into functional photoreceptors and restore light responses to the animals. These results demonstrate that hESCs can, in principle, be used for photoreceptor replacement therapies.
Figures
Similar articles
-
Derivation of traceable and transplantable photoreceptors from mouse embryonic stem cells.Stem Cell Reports. 2014 May 22;2(6):853-65. doi: 10.1016/j.stemcr.2014.04.010. eCollection 2014 Jun 3. Stem Cell Reports. 2014. PMID: 24936471 Free PMC article.
-
Inner retinal abnormalities in a mouse model of Leber's congenital amaurosis.J Comp Neurol. 2004 Feb 9;469(3):351-9. doi: 10.1002/cne.11019. J Comp Neurol. 2004. PMID: 14730587
-
Developing rods transplanted into the degenerating retina of Crx-knockout mice exhibit neural activity similar to native photoreceptors.Stem Cells. 2013 Jun;31(6):1149-59. doi: 10.1002/stem.1372. Stem Cells. 2013. PMID: 23495178 Free PMC article.
-
Cellular strategies for retinal repair by photoreceptor replacement.Prog Retin Eye Res. 2015 May;46:31-66. doi: 10.1016/j.preteyeres.2015.01.003. Epub 2015 Feb 7. Prog Retin Eye Res. 2015. PMID: 25660226 Review.
-
Organoid technology for retinal repair.Dev Biol. 2018 Jan 15;433(2):132-143. doi: 10.1016/j.ydbio.2017.09.028. Epub 2017 Dec 25. Dev Biol. 2018. PMID: 29291970 Review.
Cited by
-
The effect of retinal scaffold modulus on performance during surgical handling.Exp Eye Res. 2021 Jun;207:108566. doi: 10.1016/j.exer.2021.108566. Epub 2021 Apr 7. Exp Eye Res. 2021. PMID: 33838142 Free PMC article.
-
Thyroid hormone signaling specifies cone photoreceptor subtypes during eye development: Insights from model organisms and human stem cell-derived retinal organoids.Vitam Horm. 2021;116:51-90. doi: 10.1016/bs.vh.2021.03.001. Epub 2021 Mar 10. Vitam Horm. 2021. PMID: 33752828 Free PMC article.
-
Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice.Sci Rep. 2016 Jul 13;6:29784. doi: 10.1038/srep29784. Sci Rep. 2016. PMID: 27405580 Free PMC article.
-
Recent Progress in Photoreceptor Cell-Based Therapy for Degenerative Retinal Disease.Stem Cells Transl Med. 2024 Apr 15;13(4):332-345. doi: 10.1093/stcltm/szae005. Stem Cells Transl Med. 2024. PMID: 38417110 Free PMC article. Review.
-
Derivation of human differential photoreceptor-like cells from the iris by defined combinations of CRX, RX and NEUROD.PLoS One. 2012;7(4):e35611. doi: 10.1371/journal.pone.0035611. Epub 2012 Apr 25. PLoS One. 2012. PMID: 22558175 Free PMC article.
References
-
- Anchan RM, Reh TA, Angello J, Balliet A, Walker M. EGF and TGF-alpha stimulate retinal neuroepithelial cell proliferation in vitro. Neuron. 1991;6:923–936. - PubMed
-
- Banin E, Obolensky A, Idelson M, Hemo I, Reinhardtz E, Pikarsky E, Ben-Hur T, Reubinoff B. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006;24:246–257. - PubMed
-
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. - PubMed
-
- Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

