The G140S mutation in HIV integrases from raltegravir-resistant patients rescues catalytic defect due to the resistance Q148H mutation
- PMID: 19129221
- PMCID: PMC2651800
- DOI: 10.1093/nar/gkn1050
The G140S mutation in HIV integrases from raltegravir-resistant patients rescues catalytic defect due to the resistance Q148H mutation
Abstract
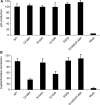
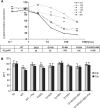
Raltegravir (MK-0518) is the first integrase (IN) inhibitor to be approved by the US FDA and is currently used in clinical treatment of viruses resistant to other antiretroviral compounds. Virological failure of Raltegravir treatment is associated with mutations in the IN gene following two main distinct genetic pathways involving either the N155 or Q148 residue. Importantly, in most cases, an additional mutation at the position G140 is associated with the Q148 pathway. Here, we investigated the viral DNA kinetics for mutants identified in Raltegravir-resistant patients. We found that (i) integration is impaired for Q148H when compared with the wild-type, G140S and G140S/Q148H mutants; and (ii) the N155H and G140S mutations confer lower levels of resistance than the Q148H mutation. We also characterized the corresponding recombinant INs properties. Enzymatic performances closely parallel ex vivo studies. The Q148H mutation 'freezes' IN into a catalytically inactive state. By contrast, the conformational transition converting the inactive form into an active form is rescued by the G140S/Q148H double mutation. In conclusion, the Q148H mutation is responsible for resistance to Raltegravir whereas the G140S mutation increases viral fitness in the G140S/Q148H context. Altogether, these results account for the predominance of G140S/Q148H mutants in clinical trials using Raltegravir.
Figures
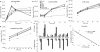


References
-
- Pommier Y, Johnson AA, Marchand C. Integrase inhibitors to treat HIV/AIDS. Nat. Rev. Drug Discov. 2005;4:236–248. - PubMed
-
- DeJesus E, Berger D, Markowitz M, Cohen C, Hawkins T, Ruane P, Elion R, Farthing C, Zhong L, Cheng AK, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J. Acquir. Immun. Defic. Syndr. 2006;43:1–5. - PubMed
-
- Hazuda DJ, Young SD, Guare JP, Anthony NJ, Gomez RP, Wai JS, Vacca JP, Handt L, Motzel SL, Klein HJ, et al. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science. 2004;305:528–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

