A simple quantitative method for analyzing electrographic status epilepticus in rats
- PMID: 19129295
- PMCID: PMC2666396
- DOI: 10.1152/jn.91062.2008
A simple quantitative method for analyzing electrographic status epilepticus in rats
Abstract
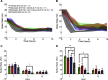
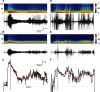
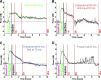
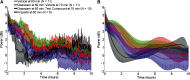
Electrographic status epilepticus (ESE) is a medical emergency consisting of repetitive seizures and may result in death or severe brain damage. Epilepsy can develop following ESE. The properties of ESE (e.g., duration and intensity) are variable, as are the effects of putative therapeutic treatments. Therefore a straightforward method to quantify different components of ESE would be beneficial for both researchers and clinicians. A frequency range close to the gamma band was selected for extraction of seizure-related activity from the EEG. This filtering strategy reduced motion artifacts and other noise sources in the electrophysiological recordings, thus increasing the signal-to-noise ratio of the EEG spike activity. EEG spiking was quantified using an energy operator and modeled by an eighth-order polynomial. In a benzodiazepine-resistant rat model of pilocarpine-induced ESE, the efficacy of various pharmaceutical agents at suppressing ESE was analyzed with this and other methods on data collected for < or =24 h after ESE induction. This approach allows for the objective, quantitative, and rapid assessment of the effects of both short- and long-lasting pharmacological manipulations on ESE and other forms of prolonged repetitive electrical activity.
Figures




References
-
- Adeli H, Zhou Z, Dadmehr N. Analysis of EEG records in an epileptic patient using wavelet transform. J Neurosci Methods 123: 69–87, 2003. - PubMed
-
- Andersen P, Bliss TV, Skrede KK. Unit analysis of hippocampal polulation spikes. Exp Brain Res Experimentelle Hirnforschung 13: 208–221, 1971. - PubMed
-
- Andersen P, Holmqvist B, Voorhoeve PE. Entorhinal activation of dentate granule cells. Acta Physiol Scand 66: 448–460, 1966. - PubMed
-
- Arboix A, Garcia-Eroles L, Massons JB, Oliveres M, Comes E. Predictive factors of early seizures after acute cerebrovascular disease. Stroke 28: 1590–1594, 1997. - PubMed
-
- Bautista RE, Godwin S, Caro D. Incorporating abbreviated EEGs in the initial workup of patients who present to the emergency room with mental status changes of unknown etiology. J Clin Neurophysiol 24: 16–21, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

