Divergent role of donor dendritic cells in rejection versus tolerance of allografts
- PMID: 19129312
- PMCID: PMC2653678
- DOI: 10.1681/ASN.2008040377
Divergent role of donor dendritic cells in rejection versus tolerance of allografts
Abstract
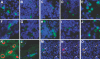
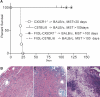
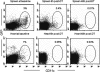
Little is known about heart tissue/donor dendritic cells, which play a key role in mounting alloimmune responses. In this report, we focus on three primary features of donor dendritic cells: their generation, their trafficking after transplantation, and their role in regulating tolerance versus rejection. Using transgenic mice as donors of heart allografts enabled us to monitor trafficking of donor dendritic cells after transplantation. Donor dendritic cells rapidly migrated into secondary lymphoid tissues within 3 h of transplantation. We found that the chemokine receptor CX3CR1 regulates the generation of heart tissue dendritic cells constitutively. Compared with wild-type hearts, CX3CR1(-/-) hearts contained fewer dendritic cells, and heart allografts from CX3CR1(-/-) donors survived significantly longer without immunosuppression. Unexpectedly, though, co-stimulatory blockade with anti-CD154 or CTLA4-Ig induced long-term survival for wild-type heart allografts but not for CX3CR1(-/-) heart allografts. Increasing the dendritic cell frequency in CX3CR1(-/-) hearts by treatment with Flt3L restored the anti-CD154-induced prolongation of CX3CR1(-/-) heart allograft survival. Compared with wild-type donors, depleting transgenic donors of dendritic cells before heart transplantation also markedly worsened chronic rejection under anti-CD154 treatment. These data indicate the importance of the CX3CR1 pathway in the generation of heart tissue dendritic cells and the divergent role of tissue/dendritic cells in rejection versus tolerance.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

