Phloem loading strategies in three plant species that transport sugar alcohols
- PMID: 19129415
- PMCID: PMC2649384
- DOI: 10.1104/pp.108.134791
Phloem loading strategies in three plant species that transport sugar alcohols
Abstract
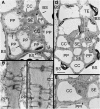
Many plants translocate sugar alcohols in the phloem. However, the mechanism(s) of sugar alcohol loading in the minor veins of leaves are debated. We characterized the loading strategies of two species that transport sorbitol (Plantago major and apple [Malus domestica]), and one that transports mannitol (Asarina scandens). Plasmodesmata are abundant at all interfaces in the minor vein phloem of apple, and in one of two types of phloem in the minor veins of A. scandens. Few plasmodesmata are present in the minor veins of P. major. Apple differs from the other two species in that sugar alcohol and sucrose (Suc) are present in much higher concentrations in leaves. Apple leaf tissue exposed to exogenous [(14)C]sorbitol, [(14)C]Suc, or (14)CO(2) did not accumulate radiolabel in the minor veins, as determined by macroautoradiography. P. major minor veins accumulated radiolabel from [(14)C]Suc, [(14)C]sorbitol, and (14)CO(2). A. scandens minor veins accumulated (14)C from [(14)C]Suc and (14)CO(2), but not from [(14)C]mannitol. We conclude that the movement of sugar alcohol from the mesophyll into the phloem in apple and A. scandens is symplastic and passive, but in P. major it involves an apoplastic step and is energized. We also suggest that apple leaves transport sorbitol in high concentrations to avoid the feedback limitation of photosynthesis that would result from driving passive movement of solute into the phloem with high levels of Suc alone. The loading pathways and the mechanisms by which hydrostatic pressure is maintained in the minor vein phloem of these species are discussed.
Figures



References
-
- Behnke HD, Sjolund RD (1990) Sieve Elements: Comparative Structure, Induction and Development. Springer-Verlag, New York
-
- Cheng L, Zhou R, Reidel EJ, Sharkey TD, Dandekar AM (2005) Antisense inhibition of sorbitol synthesis leads to up-regulation of starch synthesis without altering CO2 assimilation in apple leaves. Planta 220 767–776 - PubMed
-
- Daie J (1987) Sucrose uptake in isolated phloem of celery is a single saturable transport system. Planta 171 472–482 - PubMed
-
- Eschrich W, Fromm J (1994) Evidence for two pathways of phloem loading. Physiol Plant 90 699–707
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

