Centrosome/spindle pole-associated protein regulates cytokinesis via promoting the recruitment of MyoGEF to the central spindle
- PMID: 19129481
- PMCID: PMC2649278
- DOI: 10.1091/mbc.e08-01-0001
Centrosome/spindle pole-associated protein regulates cytokinesis via promoting the recruitment of MyoGEF to the central spindle
Abstract
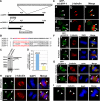
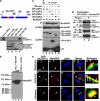
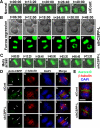
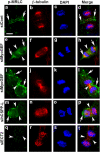
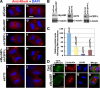
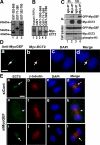
Cooperative communications between the central spindle and the contractile ring are critical for the spatial and temporal regulation of cytokinesis. Here we report that MyoGEF, a guanine nucleotide exchange factor that localizes to the central spindle and cleavage furrow, interacts with centrosome/spindle pole-associated protein (CSPP), which is concentrated at the spindle pole and central spindle during mitosis and cytokinesis. Both in vitro and in vivo pulldown assays show that MyoGEF interacts with CSPP. The C-terminus of MyoGEF and N-terminus of CSPP are required for their interaction. Immunofluorescence analysis indicates that MyoGEF and CSPP colocalize at the central spindle. Depletion of CSPP or MyoGEF by RNA-interference (RNAi) not only causes defects in mitosis and cytokinesis, such as metaphase arrest and furrow regression, but also mislocalization of nonmuscle myosin II with a phosphorylated myosin regulatory light chain (p-MRLC). Importantly, CSPP depletion by RNAi interferes with MyoGEF localization at the central spindle. Finally, MyoGEF interacts with ECT2, and RNAi-mediated depletion of MyoGEF leads to mislocalization of ECT2 and RhoA during cytokinesis. Therefore, we propose that CSPP interacts with and recruits MyoGEF to the central spindle, where MyoGEF contributes to the spatiotemporal regulation of cytokinesis.
Figures


References
-
- Burgess D. R., Chang F. Site selection for the cleavage furrow at cytokinesis. Trends Cell Biol. 2005;15:156–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

