Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis
- PMID: 19129498
- PMCID: PMC2630081
- DOI: 10.1073/pnas.0811757106
Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis
Abstract
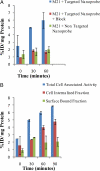
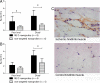
A biodegradable positron-emitting dendritic nanoprobe targeted at alpha(v)beta(3) integrin, a biological marker known to modulate angiogenesis, was developed for the noninvasive imaging of angiogenesis. The nanoprobe has a modular multivalent core-shell architecture consisting of a biodegradable heterobifunctional dendritic core chemoselectively functionalized with heterobifunctional polyethylene oxide (PEO) chains that form a protective shell, which imparts biological stealth and dictates the pharmacokinetics. Each of the 8 branches of the dendritic core was functionalized for labeling with radiohalogens. Placement of radioactive moieties at the core was designed to prevent in vivo dehalogenation, a potential problem for radiohalogens in imaging and therapy. Targeting peptides of cyclic arginine-glycine-aspartic acid (RGD) motifs were installed at the terminal ends of the PEO chains to enhance their accessibility to alpha(v)beta(3) integrin receptors. This nanoscale design enabled a 50-fold enhancement of the binding affinity to alpha(v)beta(3) integrin receptors with respect to the monovalent RGD peptide alone, from 10.40 nM to 0.18 nM IC(50). Cell-based assays of the (125)I-labeled dendritic nanoprobes using alpha(v)beta(3)-positive cells showed a 6-fold increase in alpha(v)beta(3) receptor-mediated endocytosis of the targeted nanoprobe compared with the nontargeted nanoprobe, whereas alpha(v)beta(3)-negative cells showed no enhancement of cell uptake over time. In vivo biodistribution studies of (76)Br-labeled dendritic nanoprobes showed excellent bioavailability for the targeted and nontargeted nanoprobes. In vivo studies in a murine hindlimb ischemia model for angiogenesis revealed high specific accumulation of (76)Br-labeled dendritic nanoprobes targeted at alpha(v)beta(3) integrins in angiogenic muscles, allowing highly selective imaging of this critically important process.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


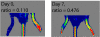

References
-
- Couvreur P, Vauthier C. Nanotechnology: Intelligent design to treat complex disease. Pharmacol Res. 2006;23:1417–1450. - PubMed
-
- Dobrucki LW, Sinusas AJ. Imaging angiogenesis. Curr Opin Biotechnol. 2007;18:90–96. - PubMed
-
- Sinusas AJ. Imaging of angiogenesis. J Nucl Cardiol. 2004;11:617–633. - PubMed
-
- Carlson CB, Mowery P, Owen RM, Dykhuizen EC, Kiessling LL. Selective tumor cell targeting using low-affinity, multivalent interactions. ACS Chem Biol. 2007;2:119–127. - PubMed
-
- Dobrucki LW, Sinusas AJ. Molecular imaging — new approach to nuclear cardiology. Q J Nucl Med Mol Imaging. 2005;49:106–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

