Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury
- PMID: 19129795
- PMCID: PMC2656389
- DOI: 10.1038/ki.2008.648
Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury
Abstract
During renal ischemia-reperfusion, local and distant tissue injury is caused by an influx of neutrophils into the affected tissues. Here we measured the kinetics of margination and transmigration of neutrophils in vivo in the kidney and lungs following renal ischemia-reperfusion. After bilateral renal injury, kidney neutrophil content increased threefold at 24 h. The neutrophils were found primarily in the interstitium and to a lesser degree marginated to the vascular endothelium. These interstitial neutrophils had significantly lower levels of intracellular IFN-gamma, IL-4, IL-6, and IL-10 a tendency for decreased amounts of IL-4 and TNF-alpha compared to the marginated neutrophils. Localization of the neutrophils to the kidney interstitium was confirmed by high resolution microscopy and these sites of transmigration were directly associated with areas of increased vascular permeability. Activation of the adenosine 2A receptor significantly decreased both kidney neutrophil transmigration by about half and vascular permeability by about a third. After unilateral renal ischemia-reperfusion, the unclipped kidney and lungs did not accumulate interstitial neutrophils or have increased vascular permeability despite a marked increase of neutrophil margination in the lungs. Our findings suggest there is a sequential recruitment and transmigration of neutrophils from the vasculature into the kidney interstitium at the site of tissue injury following renal ischemia-reperfusion.
Figures

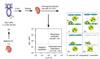






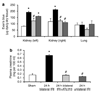
Comment in
-
Neutrophils in acute kidney injury: not neutral any more.Kidney Int. 2009 Apr;75(7):674-6. doi: 10.1038/ki.2008.689. Kidney Int. 2009. PMID: 19282858 Review.
References
-
- Li L, Okusa MD. Blocking the Immune respone in ischemic acute kidney injury: the role of adenosine 2A agonists. Nat Clin Pract Nephrol. 2006;2:432–444. - PubMed
-
- Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. - PubMed
-
- Li L, Huang L, Sung SJ, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007;178:5899–5911. - PubMed
-
- Ethuin F, Gerard B, Benna JE, et al. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–1371. - PubMed
-
- Brandt E, Woerly G, Younes AB, et al. IL-4 production by human polymorphonuclear neutrophils. J Leukoc Biol. 2000;68:125–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 DK077444/DK/NIDDK NIH HHS/United States
- 1K99DK077444-01/DK/NIDDK NIH HHS/United States
- DK56223/DK/NIDDK NIH HHS/United States
- R01 DK062324/DK/NIDDK NIH HHS/United States
- DK58413/DK/NIDDK NIH HHS/United States
- R44 DK058413/DK/NIDDK NIH HHS/United States
- P01 HL073361/HL/NHLBI NIH HHS/United States
- HL37942/HL/NHLBI NIH HHS/United States
- R41 DK058413/DK/NIDDK NIH HHS/United States
- R01 HL037942/HL/NHLBI NIH HHS/United States
- K99 DK077444/DK/NIDDK NIH HHS/United States
- DK62324/DK/NIDDK NIH HHS/United States
- R01 DK056223/DK/NIDDK NIH HHS/United States
- 1 P01 HL073361/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

