Micropit: A New Cell Culturing Approach for Characterization of Solitary Astrocytes and Small Networks of these Glial Cells
- PMID: 19129909
- PMCID: PMC2610544
- DOI: 10.3389/neuro.16.002.2008
Micropit: A New Cell Culturing Approach for Characterization of Solitary Astrocytes and Small Networks of these Glial Cells
Abstract
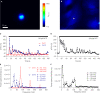
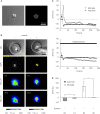
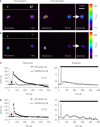
Astrocytes play an important role in cell-cell signaling in the mammalian central nervous system. The ability of astrocytes to communicate with surrounding cells through gap-junctional coupling or signaling via the release of transmitters makes characterization of these cells difficult in vitro and even more so in vivo. To simplify the complexity of common in vitro systems, introduced by intercellular communication between astrocytes, we developed a novel cell culturing method, in which purified rat visual cortical astrocytes were grown in spatially defined cell-adhesion wells which we termed micropits. We showed that astrocytes cultured in micropit regions were viable and exhibited similar characteristics of Ca(2+) dynamics and astrocytic marker expression to those of cells cultured in non-micropit regions. Examination of intracellular Ca(2+) oscillations in solitary astrocytes cultured in micropits revealed less variable oscillations than those of non-micropit grouped astrocytes, which were in contact with their neighbors. Solitary cells in micropit regions can undergo ATP-mediated astrocyte-microglia signaling, demonstrating that this culturing method can also be used to investigate glial-glial interactions in a spatially well-defined microenvironment.
Keywords: Ca2+ oscillations; astrocytes; glial–glial interactions; glutamate release; microglia.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

