Sideromycins: tools and antibiotics
- PMID: 19130258
- PMCID: PMC2757582
- DOI: 10.1007/s10534-008-9199-7
Sideromycins: tools and antibiotics
Abstract
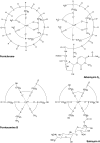
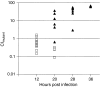
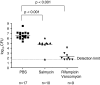
Sideromycins are antibiotics covalently linked to siderophores. They are actively transported into gram-positive and gram-negative bacteria. Energy-coupled transport across the outer membrane and the cytoplasmic membrane strongly increases their antibiotic efficiency; their minimal inhibitory concentration is at least 100-fold lower than that of antibiotics that enter cells by diffusion. This is particularly relevant for gram-negative bacteria because the outer membrane, which usually forms a permeability barrier, in this case actively contributes to the uptake of sideromycins. Sideromycin-resistant mutants can be used to identify siderophore transport systems since the mutations are usually in transport genes. Two sideromycins, albomycin and salmycin, are discussed here. Albomycin, a derivative of ferrichrome with a bound thioribosyl-pyrimidine moiety, inhibts seryl-t-RNA synthetase. Salmycin, a ferrioxamine derivative with a bound aminodisaccharide, presumably inhibts protein synthesis. Crystal structures of albomycin bound to the outer membrane transporter FhuA and the periplasmic binding protein FhuD have been determined. Albomycin and salmycin have been used to characterize the transport systems of Escherichia coli and Streptococcus pneumoniae and of Staphylococcus aureus, respectively. The in vivo efficacy of albomycin and salmycin has been examined in a mouse model using Yersinia enterocolitica, S. pneumoniae, and S. aureus infections. Albomycin is effective in clearing infections, whereas salmycin is too unstable to lead to a large reduction in bacterial numbers. The recovery rate of albomycin-resistant mutants is lower than that of the wild-type, which suggests a reduced fitness of the mutants. Albomycin could be a useful antibiotic provided sufficient quantities can be isolated from streptomycetes or synthesized chemically.
Figures

References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1054/drup.1999.0107', 'is_inner': False, 'url': 'https://doi.org/10.1054/drup.1999.0107'}, {'type': 'PubMed', 'value': '11498352', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11498352/'}]}
- Braun V (1999) Active transport of siderophore-mimicking antibacterials across the outer membrane. Drug Resist Updates 2:363–369. doi:10.1054/drup.1999.0107 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1365-2958.1993.tb01570.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1365-2958.1993.tb01570.x'}, {'type': 'PubMed', 'value': '8316079', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8316079/'}]}
- Braun V, Herrmann C (1993) Evolutionary relationship of uptake systems for biopolymers in Escherichia coli: cross-complementation between the TonB–ExbB–ExbD and the TolA–TolQ–TolR proteins. Mol Microbiol 8:261–268. doi:10.1111/j.1365-2958.1993.tb01570.x - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC215084', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC215084/'}, {'type': 'PubMed', 'value': '6352681', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/6352681/'}]}
- Braun V, Günthner K, Hantke K, Zimmermann L (1983) Intracellular activation of albomycin in Escherichia coli and Salmonella typhimurium. J Bacteriol 156:308–315 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0300-9084(02)01427-X', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0300-9084(02)01427-x'}, {'type': 'PubMed', 'value': '12423780', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12423780/'}]}
- Braun V, Patzer SI, Hantke K (2002) Ton-dependent colicins and microcins: modular design and evolution. Biochimie 84:365–380. doi:10.1016/S0300-9084(02)01427-X - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1128/IAI.70.8.4389-4398.2002', 'is_inner': False, 'url': 'https://doi.org/10.1128/iai.70.8.4389-4398.2002'}, {'type': 'PMC', 'value': 'PMC128127', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC128127/'}, {'type': 'PubMed', 'value': '12117949', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12117949/'}]}
- Brown JS, Gilliland SM, Ruiz-Albert J, Holden DW (2002) Characterization of Pit, a Streptococcus pneumoniae iron uptake ABC transporter. Infect Immun 70:4389–4398. doi:10.1128/IAI.70.8.4389-4398.2002 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

