Utilization of microbial iron assimilation processes for the development of new antibiotics and inspiration for the design of new anticancer agents
- PMID: 19130268
- PMCID: PMC4066965
- DOI: 10.1007/s10534-008-9185-0
Utilization of microbial iron assimilation processes for the development of new antibiotics and inspiration for the design of new anticancer agents
Abstract
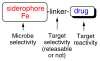
Pathogenic microbes rapidly develop resistance to antibiotics. To keep ahead in the "microbial war", extensive interdisciplinary research is needed. A primary cause of drug resistance is the overuse of antibiotics that can result in alteration of microbial permeability, alteration of drug target binding sites, induction of enzymes that destroy antibiotics (ie., beta-lactamase) and even induction of efflux mechanisms. A combination of chemical syntheses, microbiological and biochemical studies demonstrate that the known critical dependence of iron assimilation by microbes for growth and virulence can be exploited for the development of new approaches to antibiotic therapy. Iron recognition and active transport relies on the biosyntheses and use of microbe-selective iron-chelating compounds called siderophores. Our studies, and those of others, demonstrate that siderophores and analogs can be used for iron transport-mediated drug delivery ("Trojan Horse" antibiotics) and induction of iron limitation/starvation (Development of new agents to block iron assimilation). Recent extensions of the use of siderophores for the development of novel potent and selective anticancer agents are also described.
Figures







Similar articles
-
Synthetic sideromycins (skepticism and optimism): selective generation of either broad or narrow spectrum Gram-negative antibiotics.Biometals. 2019 Jun;32(3):425-451. doi: 10.1007/s10534-019-00192-6. Epub 2019 Mar 27. Biometals. 2019. PMID: 30919118
-
Design and Syntheses of New Antibiotics Inspired by Nature's Quest for Iron in an Oxidative Climate.Acc Chem Res. 2021 Apr 6;54(7):1646-1661. doi: 10.1021/acs.accounts.1c00004. Epub 2021 Mar 8. Acc Chem Res. 2021. PMID: 33684288 Free PMC article. Review.
-
Chemistry and Biology of Siderophores from Marine Microbes.Mar Drugs. 2019 Sep 29;17(10):562. doi: 10.3390/md17100562. Mar Drugs. 2019. PMID: 31569555 Free PMC article. Review.
-
High-Throughput Discovery of Synthetic Siderophores for Trojan Horse Antibiotics.ACS Infect Dis. 2024 Nov 8;10(11):3821-3841. doi: 10.1021/acsinfecdis.4c00359. Epub 2024 Oct 22. ACS Infect Dis. 2024. PMID: 39438291
-
Exploiting bacterial iron acquisition: siderophore conjugates.Future Med Chem. 2012 Mar;4(3):297-313. doi: 10.4155/fmc.11.191. Future Med Chem. 2012. PMID: 22393938 Free PMC article. Review.
Cited by
-
Antibacterial and Antibiofilm Activities of Chlorogenic Acid Against Yersinia enterocolitica.Front Microbiol. 2022 May 4;13:885092. doi: 10.3389/fmicb.2022.885092. eCollection 2022. Front Microbiol. 2022. PMID: 35602020 Free PMC article.
-
Understanding the Potential and Risk of Bacterial Siderophores in Cancer.Front Oncol. 2022 Jun 17;12:867271. doi: 10.3389/fonc.2022.867271. eCollection 2022. Front Oncol. 2022. PMID: 35785195 Free PMC article. Review.
-
Iron homeostasis--Achilles' heel of Aspergillus fumigatus?Curr Opin Microbiol. 2011 Aug;14(4):400-5. doi: 10.1016/j.mib.2011.06.002. Epub 2011 Jul 1. Curr Opin Microbiol. 2011. PMID: 21724450 Free PMC article. Review.
-
Antifungal Siderophore Conjugates for Theranostic Applications in Invasive Pulmonary Aspergillosis Using Low-Molecular TAFC Scaffolds.J Fungi (Basel). 2021 Jul 14;7(7):558. doi: 10.3390/jof7070558. J Fungi (Basel). 2021. PMID: 34356941 Free PMC article.
-
The kinase Bud32 regulates iron homeostasis in fungal pathogen Cryptococcus neoformans.Front Immunol. 2025 Jul 25;16:1624237. doi: 10.3389/fimmu.2025.1624237. eCollection 2025. Front Immunol. 2025. PMID: 40787456 Free PMC article.
References
-
- Benz G. Albomycine, I. Enzymatische Spaltung der Desferriform der Albomycine. Liebigs Ann Chem. 1984:1399–1407. doi: 10.1002/jlac.198419840802. - DOI
-
- Benz G, Schmidt D. Albomycins, 4. Isolation and total synthesis of (N-5-acetyl-N-5-hydroxy-L-Ornithyl) Liebigs Ann Chem. 1984:1434–1440. doi: 10.1002/jlac.198419840805. - DOI
-
- Benz G, Schroder T, Kurz J, Wunsche C, Karl W, Steffens G, Pfitzner J, Schmidt D. Konstitution der Desferriform der Albomycine. Angew Chem Suppl. 1982:1322–1335.
-
- Benz G, Born L, Briedan M, Grosser R, Kurz J, Paulsen H, Sinnwell V, Weber B. Albomycins, II. Absolute Konfiguration der Desferriform der Albomycine. Liebigs Ann Chem. 1984:1408–1423. doi: 10.1002/jlac.198419840803. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

