Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells
- PMID: 19130557
- PMCID: PMC2821685
- DOI: 10.1002/eji.200838232
Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells
Abstract
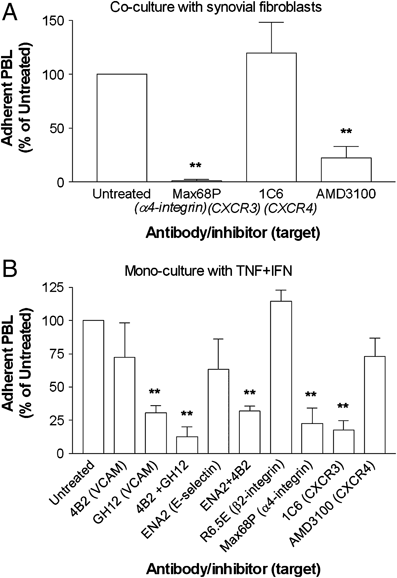
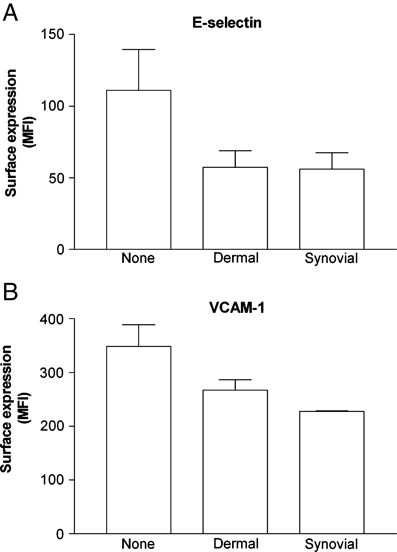
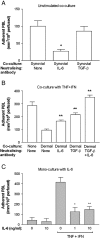
We examined the hypothesis that stromal fibroblasts modulate the ability of endothelial cells (EC) to recruit lymphocytes in a site-specific manner. PBL were perfused over HUVEC that had been cultured with fibroblasts isolated from the inflamed synovium or the skin of patients with rheumatoid arthritis or osteoarthritis, or from normal synovium, with or without exposure to the inflammatory cytokines TNF-alpha+IFN-gamma. Fibroblasts from inflamed synovium, but no others, caused unstimulated HUVEC to bind flowing lymphocytes. This adhesion was supported by alpha(4)beta(1)-VCAM-1 interaction and stabilised by activation of PBL through CXCR4-CXCL12. Antibody neutralisation of IL-6 during co-culture effectively abolished the ability of EC to bind lymphocytes. Cytokine-stimulated EC supported high levels of lymphocyte adhesion, through the presentation of VCAM-1, E-selectin and chemokine(s) acting through CXCR3. Interestingly, co-culture with dermal fibroblasts caused a marked reduction in cytokine-induced adhesion, while synovial fibroblasts had variable effects depending on their source. In the dermal co-cultures, neutralisation of IL-6 or TGF-beta caused partial recovery of cytokine-induced lymphocyte adhesion; this was complete when both were neutralised. Exogenous IL-6 was also found to inhibit response to TNF-alpha+IFN-gamma. Normal stromal fibroblasts appear to regulate the cytokine-sensitivity of vascular endothelium, while fibroblasts associated with chronic inflammation bypass this and develop a directly inflammatory phenotype. Actions of IL-6 might be pro-inflammatory or anti-inflammatory, depending on the local milieu.
Figures



Similar articles
-
Monocytes initiate a cycle of leukocyte recruitment when cocultured with endothelial cells.Atherosclerosis. 2003 Sep;170(1):49-58. doi: 10.1016/s0021-9150(03)00288-0. Atherosclerosis. 2003. PMID: 12957682
-
Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue.Arthritis Rheum. 2003 Sep;48(9):2472-82. doi: 10.1002/art.11219. Arthritis Rheum. 2003. PMID: 13130466
-
Anti-inflammatory mechanism of tocilizumab, a humanized anti-IL-6R antibody: effect on the expression of chemokine and adhesion molecule.Rheumatol Int. 2010 Jan;30(3):309-15. doi: 10.1007/s00296-009-0953-0. Epub 2009 May 23. Rheumatol Int. 2010. PMID: 19466425
-
[Rheumatoid arthritis: new developments in the pathogenesis with special reference to synovial fibroblasts].Z Rheumatol. 2001 Oct;60(5):309-18. doi: 10.1007/s003930170030. Z Rheumatol. 2001. PMID: 11759230 Review. German.
-
Endothelial cells, fibroblasts and vasculitis.Rheumatology (Oxford). 2005 Jul;44(7):860-3. doi: 10.1093/rheumatology/keh542. Epub 2005 Jan 11. Rheumatology (Oxford). 2005. PMID: 15644388 Free PMC article. Review.
Cited by
-
Augmenting endogenous repair of soft tissues with nanofibre scaffolds.J R Soc Interface. 2018 Apr;15(141):20180019. doi: 10.1098/rsif.2018.0019. J R Soc Interface. 2018. PMID: 29695606 Free PMC article. Review.
-
Analysis of the effects of stromal cells on the migration of lymphocytes into and through inflamed tissue using 3-D culture models.J Immunol Methods. 2013 Dec 31;400-401:45-57. doi: 10.1016/j.jim.2013.10.004. Epub 2013 Oct 16. J Immunol Methods. 2013. PMID: 24140419 Free PMC article.
-
Triggering the Resolution of Immune Mediated Inflammatory Diseases: Can Targeting Leukocyte Migration Be the Answer?Front Pharmacol. 2019 Mar 1;10:184. doi: 10.3389/fphar.2019.00184. eCollection 2019. Front Pharmacol. 2019. PMID: 30881306 Free PMC article.
-
Defective Induction of COX-2 Expression by Psoriatic Fibroblasts Promotes Pro-inflammatory Activation of Macrophages.Front Immunol. 2019 Mar 20;10:536. doi: 10.3389/fimmu.2019.00536. eCollection 2019. Front Immunol. 2019. PMID: 30984165 Free PMC article.
-
Two-stage implantation of the skin- and bone-integrated pylon seeded with autologous fibroblasts induced into osteoblast differentiation for direct skeletal attachment of limb prostheses.J Biomed Mater Res A. 2014 Sep;102(9):3033-48. doi: 10.1002/jbm.a.34969. Epub 2013 Oct 9. J Biomed Mater Res A. 2014. PMID: 24115308 Free PMC article.
References
-
- Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995;57:827–872. - PubMed
-
- Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. - PubMed
-
- Nash GB, Buckley CD, Rainger GE. The local physicochemical environment conditions the proinflammatory response of endothelial cells and thus modulates leukocyte recruitment. FEBS Lett. 2004;569:13–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

