Pain and inflammatory bowel disease
- PMID: 19130619
- PMCID: PMC3180862
- DOI: 10.1002/ibd.20848
Pain and inflammatory bowel disease
Abstract
Abdominal pain is a common symptom of inflammatory bowel disease (IBD: Crohn's disease, ulcerative colitis). Pain may arise from different mechanisms, which can include partial blockage and gut distention as well as severe intestinal inflammation. A majority of patients suffering from acute flares of IBD will experience pain, which will typically improve as disease activity decreases. However, a significant percentage of IBD patients continue experiencing symptoms of pain despite resolving inflammation and achieving what appears to be clinical remission. Current evidence suggests that sensory pathways sensitize during inflammation, leading to persistent changes in afferent neurons and central nervous system pain processing. Such persistent pain is not only a simple result of sensory input. Pain processing and even the activation of sensory pathways is modulated by arousal, emotion, and cognitive factors. Considering the high prevalence of iatrogenic as well as essential neuropsychiatric comorbidities including anxiety and depression in IBD patients, these central modulating factors may significantly contribute to the clinical manifestation of chronic pain. The improved understanding of peripheral and central pain mechanisms is leading to new treatment strategies that view pain as a biopsychosocial problem. Thus, improving the underlying inflammation, decreasing the excitability of sensitized afferent pathways, and altering emotional and/or cognitive functions may be required to more effectively address the difficult and disabling disease manifestations.
Figures
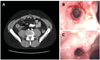



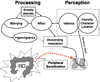
Comment in
-
Is chronic pain an extraintestinal manifestation of IBD?Inflamm Bowel Dis. 2009 May;15(5):769-71. doi: 10.1002/ibd.20844. Inflamm Bowel Dis. 2009. PMID: 19107773 No abstract available.
References
-
- Aghazadeh R, Zali MR, Bahari A, et al. Inflammatory bowel disease in Iran: a review of 457 cases. J Gastroenterol Hepatol. 2005;20:1691–1695. - PubMed
-
- Wagtmans MJ, Verspaget HW, Lamers CB, et al. Crohn’s disease in the elderly: a comparison with young adults. J Clin Gastroenterol. 1998;27:129–133. - PubMed
-
- Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol. 2005;100:2225–2229. - PubMed
-
- Edwards JT, Radford-Smith GL, Florin TH. Chronic narcotic use in inflammatory bowel disease patients: prevalence and clinical characteristics. J Gastroenterol Hepatol. 2001;16:1235–1238. - PubMed
-
- Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–630. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
