Distinct regions within the erlins are required for oligomerization and association with high molecular weight complexes
- PMID: 19131330
- PMCID: PMC2658070
- DOI: 10.1074/jbc.M809127200
Distinct regions within the erlins are required for oligomerization and association with high molecular weight complexes
Abstract
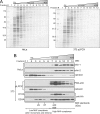
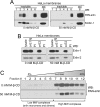
The group of stomatin/prohibitin/flotillin/HflK/C (SPFH) domain-containing proteins comprise members of diverse subcellular localization and function. Association with detergent-resistant membranes (DRMs) and the propensity to form oligomers are two common properties of SPFH domain proteins and likely important for the function of these proteins. Our laboratory recently discovered two novel members of this protein group, which, based on their endoplasmic reticulum (ER) localization and association with DRMs, were named ER lipid raft-associated protein (erlin)-1 and -2. Here we characterized erlin oligomerization and identified domains within the erlins responsible for oligomerization and DRM association. Using co-immunoprecipitation and sucrose density gradient centrifugation approaches on endogenous and ectopically expressed erlin proteins, we found that they formed homo- and hetero-oligomers and were part of large multimeric complexes. These properties were independent of their DRM association. By analyzing truncation and point mutants of erlin-2 we discovered that interaction between erlin monomers (oligomerization) and association with high molecular weight complexes require distinct regions within the protein. Although oligomerization and DRM association were mediated by a region immediately downstream of the SPFH domain (residues 228-300), integration into high molecular weight complexes was absolutely dependent on a phenylalanine residue C-terminal of this region (Phe-305), which lies within a short stretch of hydrophobic residues. Our data demonstrate that lower order oligomerization and incorporation into multimeric complexes are two separate biochemical properties of the erlins, because they are mediated by distinct regions.
Figures
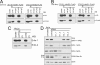


References
-
- Tavernarakis, N., Driscoll, M., and Kyrpides, N. C. (1999) Trends Biochem. Sci. 24 425-427 - PubMed
-
- Browman, D. T., Hoegg, M. B., and Robbins, S. M. (2007) Trends Cell Biol. 17 394-402 - PubMed
-
- Morrow, I. C., and Parton, R. G. (2005) Traffic 6 725-740 - PubMed
-
- Huber, T. B., Schermer, B., Muller, R. U., Hohne, M., Bartram, M., Calixto, A., Hagmann, H., Reinhardt, C., Koos, F., Kunzelmann, K., Shirokova, E., Krautwurst, D., Harteneck, C., Simons, M., Pavenstadt, H., Kerjaschki, D., Thiele, C., Walz, G., Chalfie, M., and Benzing, T. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 17079-17086 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

