Measurement of membrane-bound human heme oxygenase-1 activity using a chemically defined assay system
- PMID: 19131520
- PMCID: PMC2680543
- DOI: 10.1124/dmd.108.025023
Measurement of membrane-bound human heme oxygenase-1 activity using a chemically defined assay system
Abstract
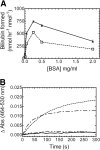
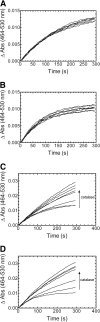
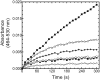
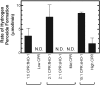
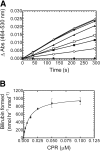
Heme oxygenase (HO) catalyzes heme degradation in a reaction requiring NADPH-cytochrome P450 reductase (CPR). Although most studies with HO used a soluble 30-kDa form, lacking the C-terminal membrane-binding region, recent reports show that the catalytic behavior of this enzyme is very different if this domain is retained; the overall activity was elevated 5-fold, and the K(m) for CPR decreased approximately 50-fold. The goal of these studies was to accurately measure HO activity using a coupled assay containing purified biliverdin reductase (BVR). This allows measurement of bilirubin formation after incorporation of full-length CPR and heme oxygenase-1 (HO-1) into a membrane environment. When rat liver cytosol was used as the source of partially purified BVR, the reaction remained linear for 2 to 3 min; however, the reaction was only linear for 10 to 30 s when an equivalent amount of purified, human BVR (hBVR) was used. This lack of linearity was not observed with soluble HO-1. Optimal formation of bilirubin was achieved with concentrations of bovine serum albumin (0.25 mg/ml) and hBVR (0.025-0.05 microM), but neither supplement increased the time that the reaction remained linear. Various concentrations of superoxide dismutase had no effect on the reaction; however, when catalase was included, the reactions were linear for at least 4 to 5 min, even at high CPR levels. These results not only show that HO-1-generated hydrogen peroxide leads to a decrease in HO-1 activity but also provide for a chemically defined system to be used to examine the function of full-length HO-1 in a membrane environment.
Figures




References
-
- Alam J (2002) Heme oxygenase-1: past, present, and future. Antioxid Redox Signal 4 559–562. - PubMed
-
- 'Carra PO and Colleran E (1969) HAEM catabolism and coupled oxidation of haemproteins. FEBS Lett 5 295–298. - PubMed
-
- Causey KM, Eyer CS, and Backes WL (1990) Dual role of phospholipid in the reconstitution of cytochrome P-450 LM2-dependent activities. Mol Pharmacol 38 134–142. - PubMed
-
- He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, and Alam J (2001) Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem 276 20858–20865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

