Physiologically based pharmacokinetics of matrine in the rat after oral administration of pure chemical and ACAPHA
- PMID: 19131523
- PMCID: PMC2680535
- DOI: 10.1124/dmd.108.023788
Physiologically based pharmacokinetics of matrine in the rat after oral administration of pure chemical and ACAPHA
Abstract
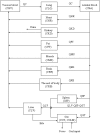
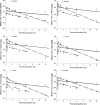
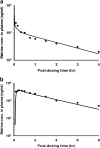
ACAPHA, a botanical drug for the treatment of human esophageal cancer in China, is under investigation as a lung cancer chemoprevention agent at the BC Cancer Agency (Vancouver, BC, Canada). Little or no information is available on the pharmacokinetics of ACAPHA in animals. The objectives of this study were as follows: to examine the disposition kinetics of matrine, a bioactive marker of ACAPHA in the rat; to develop a physiologically based pharmacokinetic (PBPK) model for pure matrine; and to characterize the absorption and clearance of crude matrine in ACAPHA-treated rats using the PBPK model. Pure matrine (15 mg/kg) or crude matrine in the form of ACAPHA (0.38 or 3.8 g/kg) was administered to the rat by gavages. The rats were sacrificed at different time points postdosing. Blood and major organs were removed from the rat, extracted with toluene/butanol, and quantified for matrine using gas chromatography-mass spectrometry. An 11-compartment, flow-limited PBPK model of matrine was developed. The PBPK model was able to simulate closely the empirical data of rats treated with pure matrine. Because the absorption and clearance of crude matrine in ACAPHA-treated rats could not be parameterized a priori, they were estimated by fitting the experimental data to the PBPK model. Results of the study show that pure matrine is absorbed and eliminated by the rat at faster rates than crude matrine. Moreover, the ACAPHA matrix may change the pharmacokinetics of matrine in the rat significantly. The PBPK model is a valuable tool to gain insights into the disposition kinetics of a botanical drug.
Figures

References
-
- Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, and Mager DE (2004) Physiological modeling of formulated and crystalline 3,3′-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos 32 632–638. - PubMed
-
- Angelo MJ and Pritchard AB (1987) Route-to-route extrapolation of dichloromethane exposure using a physiological pharmacokinetic model, in Pharmacokinetics in Risk Assessment: Drinking Water and Health, pp 254–264, National Academy Press, Washington, DC.
-
- Benowitz N, Forsyth FP, Melmon KL, and Rowland M (1974) Lidocaine disposition kinetics in monkey and man. I. Prediction by a perfusion model. Clin Pharmacol Ther 16 87–98. - PubMed
-
- Chang MY (1992) Anticancer Medicinal Herbs, Human Science and Technology Publishing House, Changsha, China.
-
- Clewell HJ and Andersen ME (1985) Risk assessment extrapolations and physiological modelling, in Advances in Health Risk Assessment for Systemic Toxicants and Chemical Mixtures: An International Symposium (Stara JF, Erdreich LS, eds), pp 111–131, Princeton Scientific Publishing Co., Princeton, NJ.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

