Nicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter gamma-aminobutyric acid
- PMID: 19131543
- PMCID: PMC2722153
- DOI: 10.1093/carcin/bgp010
Nicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter gamma-aminobutyric acid
Abstract
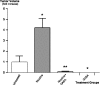
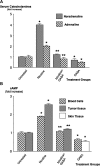
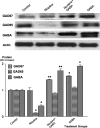
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of cancer mortality in Western countries. We have shown previously that four representative human PDAC cell lines were regulated by beta-adrenoreceptors via cyclic adenosine 3',5'-monophosphate (cAMP)-dependent signaling. In the current study, we have tested the hypothesis that nicotine stimulates the growth of PDAC xenografts in nude mice by increasing the systemic levels of the stress neurotransmitters adrenaline and noradrenaline, which are the physiological agonists for beta-adrenoreceptors and that inhibition by gamma-aminobutyric acid (GABA) of the adenylyl cyclase-dependent pathway downstream of adrenoreceptors blocks this effect. The size of xenografts from PDAC cell line Panc-1 was determined 30 days after inoculation of the cancer cells. Stress neurotransmitters in serum as well as cAMP in the cellular fraction of blood and in tumor tissue were assessed by immunoassays. Levels of GABA, its synthesizing enzymes GAD65 and GAD67 and beta-adrenergic signaling proteins in the tumor tissue were determined by western blotting. Nicotine significantly increased the systemic levels of adrenaline, noradrenaline and cAMP while increasing xenograft size and protein levels of cAMP, cyclic AMP response element-binding protein and p-extracellular signal-regulated kinase 1/2 in the tumor tissue. Nicotine additionally reduced the protein levels of both GAD isozymes and GABA in tumor tissue. Treatment with GABA abolished these responses to nicotine and blocked the development of xenografts in mice not exposed to nicotine. These findings suggest that the development and progression of PDAC is subject to significant modulation by stimulatory stress neurotransmitters and inhibitory GABA and that treatment with GABA may be useful for marker-guided cancer intervention of PDAC.
Figures

References
-
- Mancuso A, et al. Current therapies and advances in the treatment of pancreatic cancer. Crit. Rev. Oncol. Hematol. 2006;58:231–241. - PubMed
-
- Lowenfels AB, et al. Risk factors for pancreatic cancer. J. Cell. Biochem. 2005;95:649–656. - PubMed
-
- Nieto J, et al. Metastatic pancreatic cancer 2008: is the glass less empty? Oncologist. 2008;13:562–576. - PubMed
-
- Wadler S. Molecular targeting in pancreatic cancer. Rev. Recent Clin. Trials. 2007;2:69–75. - PubMed
-
- Rall TW. Drugs used in the treatment of asthma. In: Goodman-Gilman A, Rall TW, Nies AS, Taylor P, editors. The Pharmacologiccal Basis of Therapeutics. New York, NY: Pergamon Press; 1990. pp. 618–637.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

