Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1
- PMID: 19131551
- PMCID: PMC2651010
- DOI: 10.1182/blood-2008-09-178459
Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1
Abstract
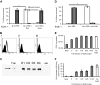
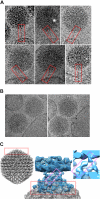
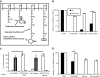
Type 5 adenovirus (Ad5) is a human pathogen that has been widely developed for therapeutic uses, with only limited success to date. We report here the novel finding that human erythrocytes present Coxsackie virus-adenovirus receptor (CAR) providing an Ad5 sequestration mechanism that protects against systemic infection. Interestingly, erythrocytes from neither mice nor rhesus macaques present CAR. Excess Ad5 fiber protein or anti-CAR antibody inhibits the binding of Ad5 to human erythrocytes and cryo-electron microscopy shows attachment via the fiber protein of Ad5, leading to close juxtaposition with the erythrocyte membrane. Human, but not murine, erythrocytes also present complement receptor (CR1), which binds Ad5 in the presence of antibodies and complement. Transplantation of human erythrocytes into nonobese diabetic/severe combined immunodeficiency mice extends blood circulation of intravenous Ad5 but decreases its extravasation into human xenograft tumors. Ad5 also shows extended circulation in transgenic mice presenting CAR on their erythrocytes, although it clears rapidly in transgenic mice presenting erythrocyte CR1. Hepatic infection is inhibited in both transgenic models. Erythrocytes may therefore restrict Ad5 infection (natural and therapeutic) in humans, independent of antibody status, presenting a formidable challenge to Ad5 therapeutics. "Stealthing" of Ad5 using hydrophilic polymers may enable circumvention of these natural virus traps.
Figures


References
-
- Parker AL, Waddington SN, Nicol CG, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. - PubMed
-
- Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. - PubMed
-
- Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16:1016–1027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

