Common and distinct genetic properties of ESCRT-II components in Drosophila
- PMID: 19132102
- PMCID: PMC2613530
- DOI: 10.1371/journal.pone.0004165
Common and distinct genetic properties of ESCRT-II components in Drosophila
Abstract
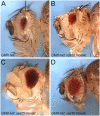
Background: Genetic studies in yeast have identified class E vps genes that form the ESCRT complexes required for protein sorting at the early endosome. In Drosophila, mutations of the ESCRT-II component vps25 cause endosomal defects leading to accumulation of Notch protein and increased Notch pathway activity. These endosomal and signaling defects are thought to account for several phenotypes. Depending on the developmental context, two different types of overgrowth can be detected. Tissue predominantly mutant for vps25 displays neoplastic tumor characteristics. In contrast, vps25 mutant clones in a wild-type background trigger hyperplastic overgrowth in a non-autonomous manner. In addition, vps25 mutant clones also promote apoptotic resistance in a non-autonomous manner.
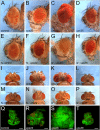
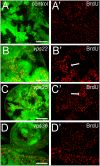
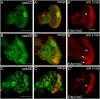
Principal findings: Here, we genetically characterize the remaining ESCRT-II components vps22 and vps36. Like vps25, mutants of vps22 and vps36 display endosomal defects, accumulate Notch protein and--when the tissue is predominantly mutant--show neoplastic tumor characteristics. However, despite these common phenotypes, they have distinct non-autonomous phenotypes. While vps22 mutations cause strong non-autonomous overgrowth, they do not affect apoptotic resistance. In contrast, vps36 mutations increase apoptotic resistance, but have little effect on non-autonomous proliferation. Further characterization reveals that although all ESCRT-II mutants accumulate Notch protein, only vps22 and vps25 mutations trigger Notch activity.
Conclusions/significance: The ESCRT-II components vps22, vps25 and vps36 display common and distinct genetic properties. Our data redefine the role of Notch for hyperplastic and neoplastic overgrowth in these mutants. While Notch is required for hyperplastic growth, it appears to be dispensable for neoplastic transformation.
Conflict of interest statement
Figures




Similar articles
-
vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis.Development. 2006 May;133(10):1871-80. doi: 10.1242/dev.02356. Epub 2006 Apr 12. Development. 2006. PMID: 16611691 Free PMC article.
-
Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants.J Cell Sci. 2009 Jul 15;122(Pt 14):2413-23. doi: 10.1242/jcs.046391. J Cell Sci. 2009. PMID: 19571114 Free PMC article.
-
De-regulation of JNK and JAK/STAT signaling in ESCRT-II mutant tissues cooperatively contributes to neoplastic tumorigenesis.PLoS One. 2013;8(2):e56021. doi: 10.1371/journal.pone.0056021. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418496 Free PMC article.
-
Tumor suppressors: control of signaling by endocytosis.Curr Biol. 2006 Feb 7;16(3):R91-2. doi: 10.1016/j.cub.2006.01.022. Curr Biol. 2006. PMID: 16461271 Review.
-
ESCRT proteins in physiology and disease.Exp Cell Res. 2009 May 15;315(9):1619-26. doi: 10.1016/j.yexcr.2008.10.013. Epub 2008 Oct 28. Exp Cell Res. 2009. PMID: 19013455 Review.
Cited by
-
Notch signaling activates Yorkie non-cell autonomously in Drosophila.PLoS One. 2012;7(6):e37615. doi: 10.1371/journal.pone.0037615. Epub 2012 Jun 5. PLoS One. 2012. PMID: 22679484 Free PMC article.
-
Regulation of ligand-independent Notch signal through intracellular trafficking.Commun Integr Biol. 2012 Jul 1;5(4):374-6. doi: 10.4161/cib.19995. Commun Integr Biol. 2012. PMID: 23060962 Free PMC article.
-
Ligand-independent traffic of Notch buffers activated Armadillo in Drosophila.PLoS Biol. 2009 Aug;7(8):e1000169. doi: 10.1371/journal.pbio.1000169. Epub 2009 Aug 11. PLoS Biol. 2009. PMID: 19668359 Free PMC article.
-
Endocytosis and intracellular trafficking of Notch and its ligands.Curr Top Dev Biol. 2010;92:165-200. doi: 10.1016/S0070-2153(10)92005-X. Curr Top Dev Biol. 2010. PMID: 20816395 Free PMC article. Review.
-
The histone demethylase Kdm5 controls Hid-induced cell death in Drosophila.Front Cell Death. 2024;3:1471050. doi: 10.3389/fceld.2024.1471050. Epub 2024 Nov 19. Front Cell Death. 2024. PMID: 40416947
References
-
- Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 2005;6:610–621. - PubMed
-
- Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–368. - PubMed
-
- Gonzalez-Gaitan M. Signal dispersal and transduction through the endocytic pathway. Nat Rev Mol Cell Biol. 2003;4:213–224. - PubMed
-
- Le Roy C, Wrana JL. Signaling and endocytosis: a team effort for cell migration. Dev Cell. 2005;9:167–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

