Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds
- PMID: 19132705
- PMCID: PMC2803615
- DOI: 10.1002/chem.200802140
Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds
Abstract
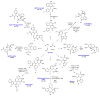
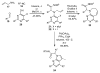
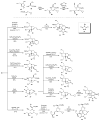
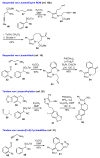
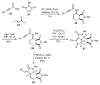
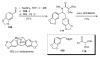
The sequencing of multicomponent reactions (MCRs) and subsequent cyclization reactions is a powerful stratagem for the rapid synthesis of diverse heterocyclic scaffolds. The optimal MCR is sufficiently flexible that it can be employed to generate adducts bearing a variety of functional groups that may then be selectively paired to enable different cyclization manifolds, thereby leading to a diverse collection of products. The growing interest in diversity-oriented synthesis has led to increased attention to this paradigm for library synthesis, which has inspired many advances in the design and implementation of MCRs for the construction of diverse heterocyclic scaffolds.
Figures
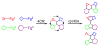
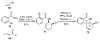





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

