Generation of DNA interstrand cross-links by post-synthetic reductive amination
- PMID: 19132933
- PMCID: PMC2635425
- DOI: 10.1021/ol802719a
Generation of DNA interstrand cross-links by post-synthetic reductive amination
Abstract
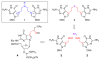
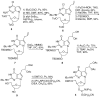
DNA interstrand cross-links (ICLs) are the clinically most relevant adducts formed by many antitumor agents. To facilitate the study of biological responses triggered by ICLs, we developed a new approach toward the synthesis of mimics of nitrogen mustard ICLs. 7-Deazaguanine residues bearing acetaldehyde groups were incorporated into complementary strands of DNA and cross-link formation induced by double reductive amination. Our strategy enables the synthesis of major groove cross-links in high yields and purity.
Figures

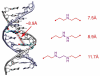
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

