Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues
- PMID: 19133304
- PMCID: PMC2667119
- DOI: 10.1016/j.addr.2008.11.002
Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues
Abstract
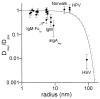
Mucus is a viscoelastic and adhesive gel that protects the lung airways, gastrointestinal (GI) tract, vagina, eye and other mucosal surfaces. Most foreign particulates, including conventional particle-based drug delivery systems, are efficiently trapped in human mucus layers by steric obstruction and/or adhesion. Trapped particles are typically removed from the mucosal tissue within seconds to a few hours depending on anatomical location, thereby strongly limiting the duration of sustained drug delivery locally. A number of debilitating diseases could be treated more effectively and with fewer side effects if drugs and genes could be more efficiently delivered to the underlying mucosal tissues in a controlled manner. This review first describes the tenacious mucus barrier properties that have precluded the efficient penetration of therapeutic particles. It then reviews the design and development of new mucus-penetrating particles that may avoid rapid mucus clearance mechanisms, and thereby provide targeted or sustained drug delivery for localized therapies in mucosal tissues.
Figures

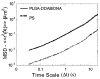



References
-
- Rosen H, Abribat T. The rise and rise of drug delivery. Nat Rev Drug Discov. 2005;4:381–5. - PubMed
-
- Shmulewitz A, Langer R, Patton J. Convergence in biomedical technology. Nat Biotech. 2006;24:277–277.
-
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–60. - PubMed
-
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22. - PubMed
-
- Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

